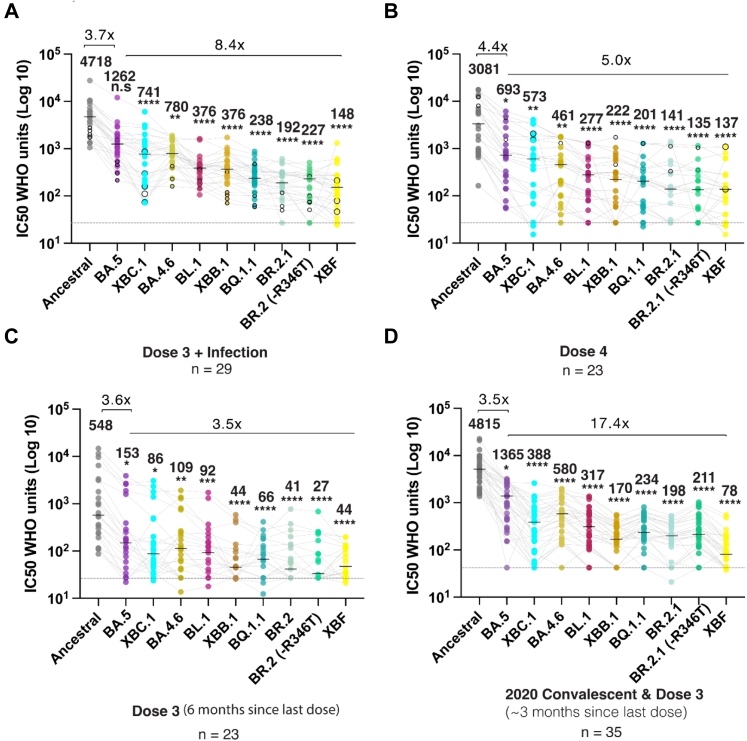
Fig. 3.
Emerging variants and their ability to evade a continuum of antibody responses. A. Three dose Pfizer BNT162b2 vaccination with subsequent breakthrough infection. Closed circles were infections between June and August 2022 when BA.2 and BA.5 were prevalent. Open circles are breakthrough infections with BA.1 between January and February. B. Primarily four dose BNT162b2 vaccinations, in which the last dose was within three months. Open circles in this group are breakthrough infections in early 2022 at the time of BA.1. C. Three dose Pfizer BNT162b2 vaccination with the last dose six months prior. D. Early 2020 donors infected between March and August of 2020 and then subsequently vaccinated with two doses of Pfizer BNT162b2 or AstraZeneca AZD1222/ChAdOx1 and boosted with Pfizer BNT162b2 or Moderna mRNA-1273. This group did not receive their last dose three to six months prior. Data in (A–D) indicates the mean IC50 of technical replicates for individual samples. The median titers are labelled. Fold change reductions in IC50 neutralization titers compare variants of concern to the Ancestral variant and Omicron BA.5 where indicated. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 for Friedman test with Dunn's multiple comparison test.