Figure 1.
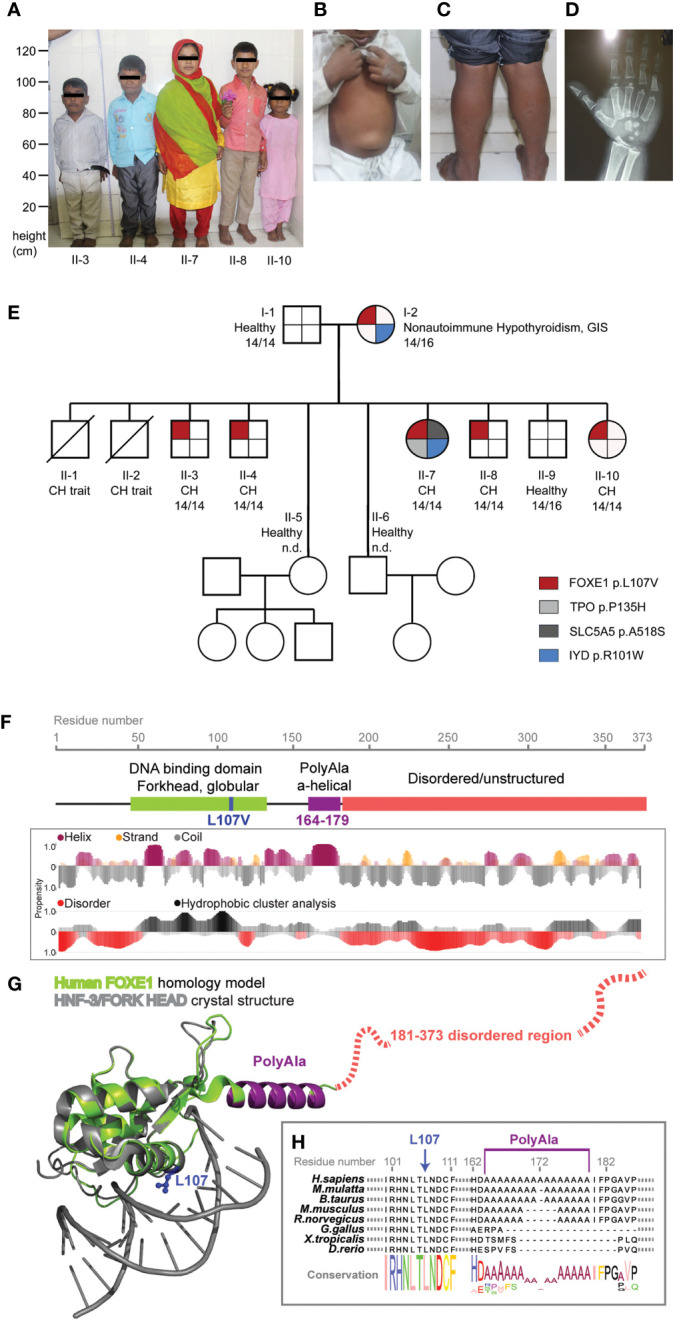
FOXE1 variants segregation in the family and protein structural modeling. (A) picture of the five affected siblings showing severe short stature, puffy facies with dry skin, sparse fragile hair, lateral madarosis and short round nose. (B) umbilical hernia in patient II-3. (C) calf muscle pseudo-hypertrophy in patient II-4. (D) X ray of left hand revealing delayed bone age and epiphyseal dysplasia in patient II-7. (E) family tree showing FOXE1 and other genetic variants co-segregation with CH (14/14, Ala-14/14 FOXE1; 14/16, Ala-14/16 FOXE1; 16/16, Ala-16/16 FOXE1; n.d., not determined). (F) linear representation of FOXE1 sequence and local secondary structure prediction adopted by the FOXE1 aminoacidic residues calculated with the software FELLS. The plot indicates the probability of a defined secondary structure or disordered coiled to be adopted per sequence residue. (G) AlphaFold in silico model of the human FOXE1 structured region (residues 56-181), superimposed with the experimental crystal structure of a homologue transcription factor bound to DNA (HNF-3/forkhead, PDB ID: 1VTN). FOXE1 model shows a conserved DNA binding domain (green), followed by a poly-alanine alpha-helical region (purple) and by and unstructured disordered region (red dotted line). The relative position of L107 residue and polyalanine tract in respect to DNA binding suggest proximity but not apparent direct binding to DNA. (H) FOXE1 alignment showing the highly conserved L107 residue in the DNA binding domain and the relatively late evolutionary emergence of the polyalanine tract.
