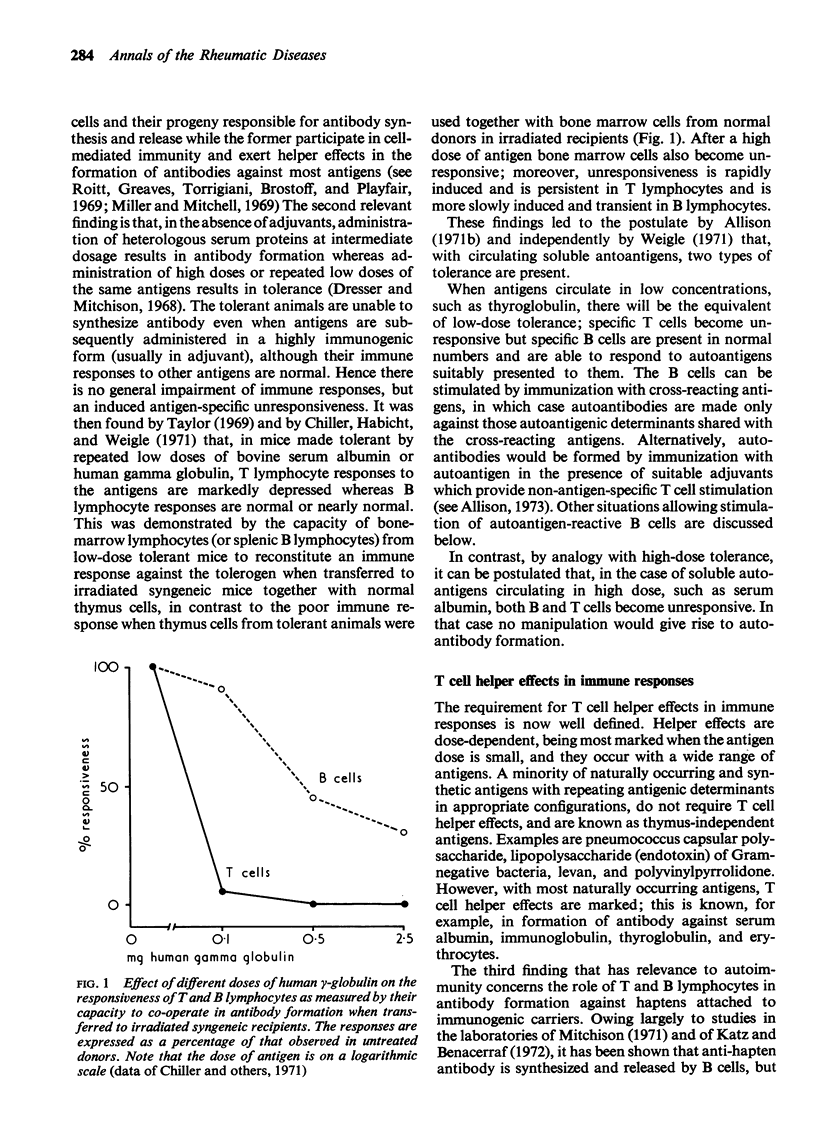
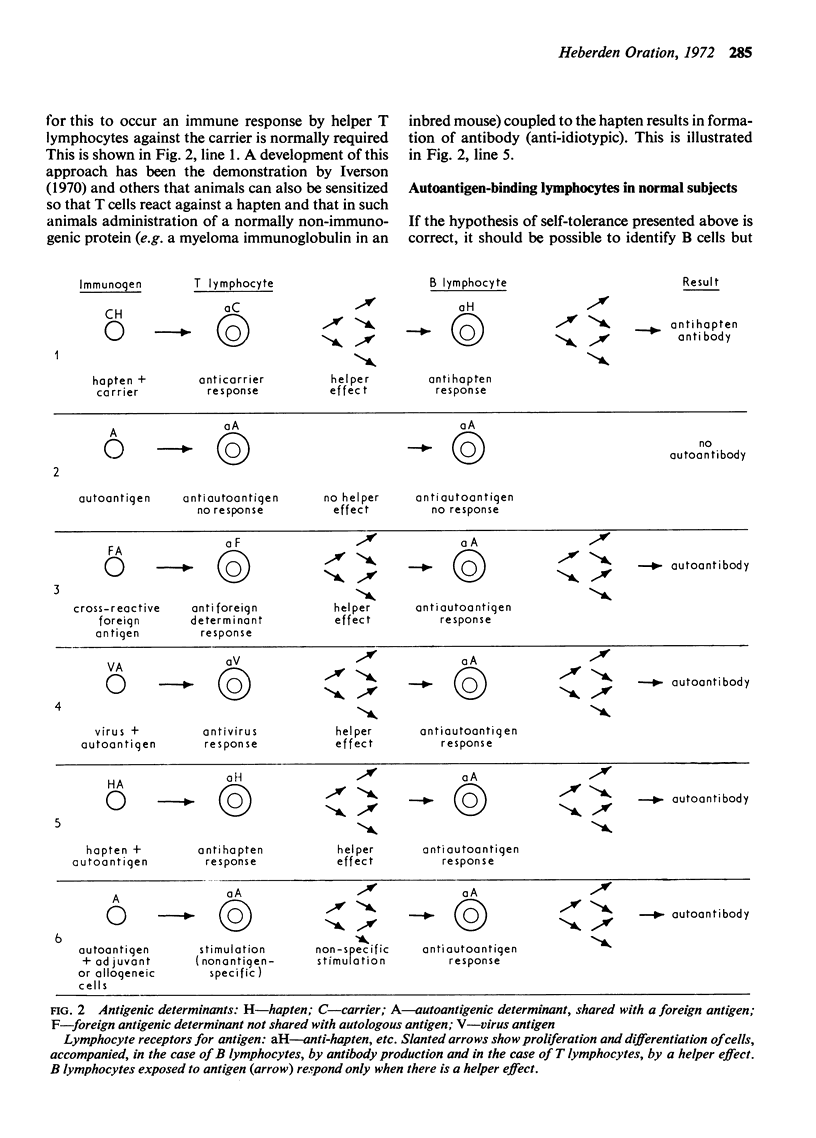
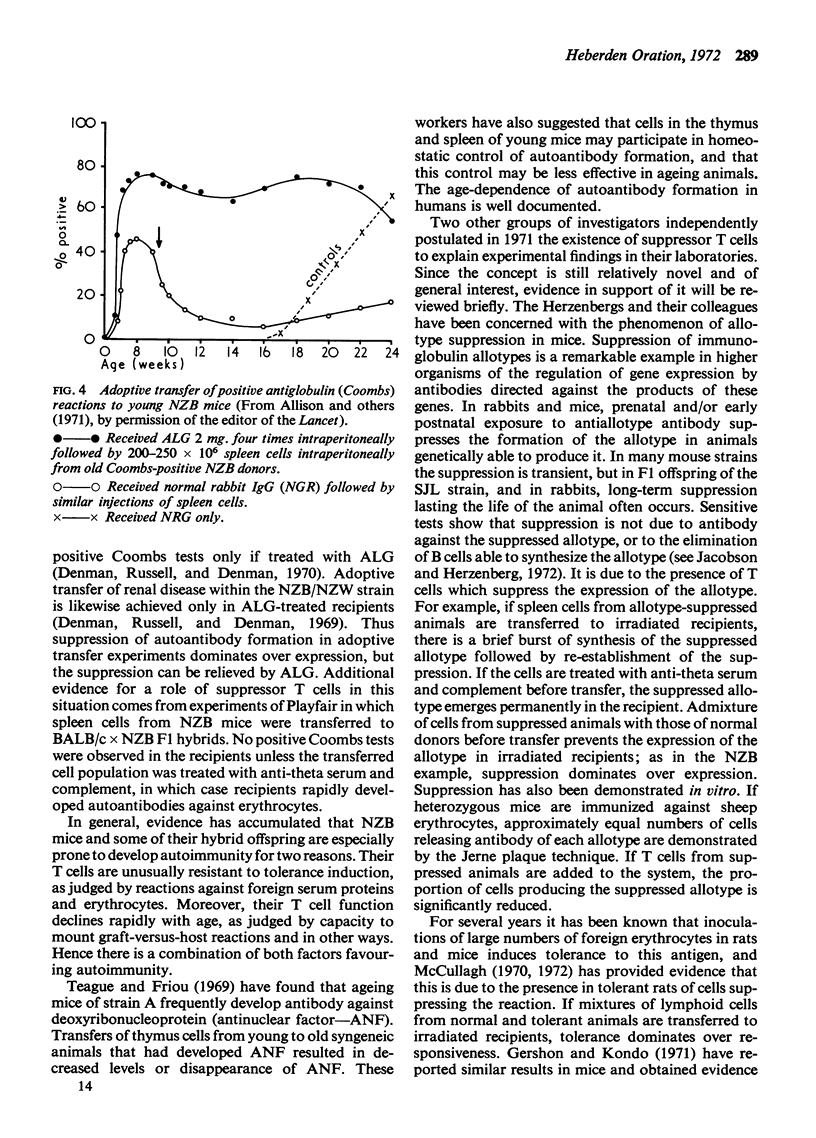
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L. Antigen binding cells in tolerance and immunity. Transplant Rev. 1970;5:105–129. doi: 10.1111/j.1600-065x.1970.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Barnes R. M. The mechanism of immunological unresponsiveness to picryl chloride and the possible role of antibody mediated depression. Clin Exp Immunol. 1971 Jul;9(1):111–121. [PMC free article] [PubMed] [Google Scholar]
- Bankhurst A. D., Torrigiani G., Allison A. C. Lymphocytes binding human thyroglobulin in healthy people and its relevance to tolerance for autoantigens. Lancet. 1973 Feb 3;1(7797):226–230. doi: 10.1016/s0140-6736(73)90066-4. [DOI] [PubMed] [Google Scholar]
- Cannat A., Seligmann M. Induction by isoniazid and hydrallazine of antinuclear factors in mice. Clin Exp Immunol. 1968 Jan;3(1):99–105. [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 1971 Feb 26;171(3973):813–815. doi: 10.1126/science.171.3973.813. [DOI] [PubMed] [Google Scholar]
- Cole G. A., Nathanson N., Prendergast R. A. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature. 1972 Aug 11;238(5363):335–337. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- Denman A. M., Russell A. S., Denman E. J. Adoptive transfer of the diseases of New Zealand black mice to normal mouse strains. Clin Exp Immunol. 1969 Dec;5(6):567–595. [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W., Mitchison N. A. The mechanism of immunological paralysis. Adv Immunol. 1968;8:129–181. doi: 10.1016/s0065-2776(08)60466-6. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Nossal G. J. Tolerance, enhancement and the regulation of interactions between T cells, B cells and macrophages. Transplant Rev. 1972;13:3–34. doi: 10.1111/j.1600-065x.1972.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Gilchrist C., Allison A. C. Autoimmunity in chronic graft-versus-host disease. Clin Exp Immunol. 1973 Apr;13(4):479–486. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Cohen P., Hencin R., Liebhaber S. A. Suppressor T cells. J Immunol. 1972 Mar;108(3):586–590. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Infectious immunological tolerance. Immunology. 1971 Dec;21(6):903–914. [PMC free article] [PubMed] [Google Scholar]
- Hannestad K., Kao M. S., Eisen H. N. Cell-bound myeloma proteins on the surface of myeloma cells: potential targets for the immune system. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2295–2299. doi: 10.1073/pnas.69.8.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. S., Allison A. C., Harvey J. J. Immune complexes in mice infected neonatally with Moloney leukaemogenic and murine sarcoma viruses. Nature. 1969 Aug 16;223(5207):739–740. doi: 10.1038/223739a0. [DOI] [PubMed] [Google Scholar]
- Iverson G. M. Ability of CBA mice to produce anti-idiotypic sera to 5563 myeloma protein. Nature. 1970 Jul 18;227(5255):273–274. doi: 10.1038/227273a0. [DOI] [PubMed] [Google Scholar]
- Jacobson E. B., Herzenberg L. A. Active suppression of immunoglobulin allotype synthesis. I. Chronic suppression after perinatal exposure to maternal antibody to paternal allotype in (SJL x BALB-c)F 1 mice. J Exp Med. 1972 May 1;135(5):1151–1162. doi: 10.1084/jem.135.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H. The allogeneic effect on immune responses: model for regulatory influences of T lymphocytes on the immune system. Transplant Rev. 1972;12:141–179. doi: 10.1111/j.1600-065x.1972.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Lindenmann J., Klein P. A. Viral oncolysis: increased immunogenicity of host cell antigen associated with influenza virus. J Exp Med. 1967 Jul 1;126(1):93–108. doi: 10.1084/jem.126.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P. J. The transfer of immunological competence to rats tolerant of sheep erythrocytes with lymphocytes from normal rats. Aust J Exp Biol Med Sci. 1970 Aug;48(4):351–367. doi: 10.1038/icb.1970.38. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Naor D., Sulitzeanu D. Binding of 125I-BSA to lymphoid cells of tolerant mice. Int Arch Allergy Appl Immunol. 1969;36(1):112–113. doi: 10.1159/000230728. [DOI] [PubMed] [Google Scholar]
- OUDIN J., MICHEL M. [A new allotype form of rabbit serum gamma-globulins, apparently associated with antibody function and specificity]. C R Hebd Seances Acad Sci. 1963 Jul 17;257:805–808. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn I., Starzl T. E. Malignant tumors arising de novo in immunosuppressed organ transplant recipients. Transplantation. 1972 Oct;14(4):407–417. doi: 10.1097/00007890-197210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseier H., Lindenmann J. F1 hybrid animals: reactivity against recognition structures of parental strain lymphoid cells. Pathol Microbiol (Basel) 1969;34(6):379–387. doi: 10.1159/000162189. [DOI] [PubMed] [Google Scholar]
- Roitt I. M., Greaves M. F., Torrigiani G., Brostoff J., Playfair J. H. The cellular basis of immunological responses. A synthesis of some current views. Lancet. 1969 Aug 16;2(7616):367–371. doi: 10.1016/s0140-6736(69)92712-3. [DOI] [PubMed] [Google Scholar]
- Teague P. O., Friou G. J. Antinuclear antibodies in mice. II. Transmission with spleen cells; inhibition or prevention with thymus or spleen cells. Immunology. 1969 Nov;17(5):665–675. [PMC free article] [PubMed] [Google Scholar]
- Tonietti G., Oldstone M. B., Dixon F. J. The effect of induced chronic viral infections on the immunologic diseases of New Zealand mice. J Exp Med. 1970 Jul 1;132(1):89–109. doi: 10.1084/jem.132.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrigiani G., Doniach D., Roitt I. M. Serum thyroglobulin levels in healthy subjects and in patients with thyroid disease. J Clin Endocrinol Metab. 1969 Mar;29(3):305–314. doi: 10.1210/jcem-29-3-305. [DOI] [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-binding cells. I. Their idenification and role in the immune response. J Immunol. 1971 Oct;107(4):1168–1174. [PubMed] [Google Scholar]
- Volkert M., Larsen J. H. Immunological tolerance to viruses. Prog Med Virol. 1965;7:160–207. [PubMed] [Google Scholar]
- Weigle W. O. Recent observations and concepts in immunological unresponsiveness and autoimmunity. Clin Exp Immunol. 1971 Oct;9(4):437–447. [PMC free article] [PubMed] [Google Scholar]
- Wick G., Kite J. H., Jr, Witebsky E. Spontaneous thyroiditis in the obese strain of chickens. IV. The effect of thymectomy and thymo-bursectomy on the development of the disease. J Immunol. 1970 Jan;104(1):54–62. [PubMed] [Google Scholar]


