Abstract
Background
Primary hypoadrenocorticism (PH) is rare in cats and knowledge about treatment is sparse.
Objective
To describe cats with PH with a focus on long‐term treatment.
Animals
Eleven cats with naturally occurring PH.
Methods
Descriptive case series with data on signalment, clinicopathological findings, adrenal width, and doses of desoxycorticosterone pivalate (DOCP) and prednisolone during a follow‐up period of >12 months.
Results
Cats ranged from 2 to 10 years (median 6.5); 6 cats were British Shorthair. Most common signs were reduced general condition and lethargy, anorexia, dehydration, obstipation, weakness, weight loss, and hypothermia. Adrenal glands on ultrasonography were judged small in 6. Eight cats could be followed for 14 to 70 months (median: 28). Two were started on DOCP doses ≥2.2 mg/kg (2.2; 2.5) and 6 < 2.2 mg/kg (1.5‐2.0 mg/kg, median 1.8) q28 days. Both high‐dose cats and 4 low‐dose cats needed a dose increase. Desoxycorticosterone pivalate and prednisolone doses at the end of the follow‐up period were 1.3 to 3.0 mg/kg (median: 2.3) and 0.08 to 0.5 mg/kg/day (median: 0.3), respectively.
Conclusions and Clinical Importance
Desoxycorticosterone pivalate and prednisolone requirements in cats were higher than what is currently used in dogs; thus, a DOCP starting dose of 2.2 mg/kg q28 days and a prednisolone maintenance dose of 0.3 mg/kg/day titrated to the individual need seems warranted. Small adrenal glands (width < 2.7 mm) on ultrasonography in a cat suspected of hypoadrenocorticism can be suggestive of the disease. The apparent predilection of British Shorthaired cats for PH should be further evaluated.
Keywords: Addison's disease, feline, mineralocorticoid, therapy
Abbreviations
- ACTH
adrenocorticotropic hormone
- CK
creatinine kinase
- DGGR
1,2‐o‐dilaurylrac‐glycero‐3‐glutaric acid‐(60‐methylresorufin) ester
- DOCP
desoxycorticosterone pivalate
- PH
primary hypoadrenocorticism
- RDVM
referring veterinarian
1. INTRODUCTION
Primary hypoadrenocorticism (PH) is a rare disease in cats. Its incidence seems lower than in dogs, even in cats presenting with hyperkalemia, hyponatremia, or a low sodium: potassium ratio. 1 So far, no breed or sex predisposition is reported and many cats are less than 5 years old at diagnosis. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 The anamnestic signs are like those in dogs and include lethargy, anorexia, weight loss, and, less commonly, vomiting, polyuria/polydipsia, and waxing/waning illness. At presentation, cats are usually lethargic, weak, dehydrated, and hypothermic. As in dogs, the most common biochemical findings in cats with PH are hyponatremia and hyperkalemia. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9
In dogs, adrenal ultrasonography can help during the diagnostic work‐up of an animal with clinical signs suggestive of PH. 10 In cats with PH, exact adrenal measurements are rarely reported; in the majority of the few cases where adrenals have been described, they were judged to be normal in size. 5 , 6 , 7 , 8 So far, there is only 1 report of a cat with PH having small adrenal glands (width 2.2 mm). 11
Most cats reported in the literature have been treated with fludrocortisone acetate. 2 , 3 , 4 , 6 , 7 Only 7 cats treated with DOCP have so far been described. 2 , 7 , 8 , 9 DOCP is a parenteral, long‐acting mineralocorticoid with no glucocorticoid activity; therefore, additional supplementation with glucocorticoids is mandatory. The manufacturer recommends a starting dose of 2.2 mg/kg every 28 days in dogs. However, several reports have shown that dogs can be stabilized on much lower doses or longer dose intervals. 12 , 13 , 14 , 15 , 16 Reported DOCP doses in the 7 cats described in the case reports mentioned above ranged from 2.2 to 2.6 mg/kg and reported intervals from 28 to 40 days. 2 , 7 , 8 , 9 The follow‐up period of these cases is not clearly mentioned in 3 or was below 12 months in 2 cases. 2 , 7 , 8 , 9
Because of the rarity of the disease, studies in larger groups of cats with PH that evaluate DOCP and the glucocorticoid treatment on a long‐term basis, as well as dosing schemes and dosing intervals are lacking. In addition, given the paucity of data regarding adrenal gland measurements in cats, we sought to report adrenal gland width in the same cohort of cats.
Therefore, the aims of the present case series were 2‐fold: first, to characterize the disease in cats by describing the signalment, clinical signs, clinicopathological findings, and ultrasonographic measurements of the adrenal glands of cats with naturally occurring PH; and second, to describe treatment and long‐term follow‐up information with an emphasis on the description of the DOCP and glucocorticoid dosage over a time period of at least 12 months.
2. MATERIALS AND METHODS
2.1. Animals
Cats presented to the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty University of Zurich, Switzerland, between January 2010 and June 2021 with naturally occurring PH were enrolled. PH was diagnosed based on a post‐ACTH serum cortisol concentration of <55 mmol/L, abnormal serum sodium and/or potassium concentrations, and/or elevated plasma endogenous ACTH concentrations. Cases in which causes of hypoadrenocorticism were iatrogenic or because of neoplasia, adrenalectomy, or trilostane treatment were excluded.
For the second part of the case series cats had to be treated with DOCP and had to go through a minimum follow‐up period of 12 months.
2.2. Analytical procedures
For the ACTH stimulation test, blood samples were taken before and 60 minutes after IV injection of 5 μg/kg synthetic ACTH (Synacthen, Future Health Pharma GmbH, Wetzikon, Switzerland). Serum cortisol concentrations were measured by a competitive immunoassay (DPC Immulite 1 (samples received until July 2011), DPC Immulite 1000 (samples from August 2011 to September 2016), DPC Immulite 2000 (samples from September 2016 until June 2021; Siemens Schweiz AG, Zurich, Switzerland). For the determination of plasma endogenous ACTH (9 cats), blood was collected (before ACTH administration) into chilled EDTA‐coated tubes placed on ice and centrifuged at 4°C within 30 minutes. Plasma ACTH concentrations were determined by a 2‐site solid‐phase chemiluminescent immunometric assay (DPC Immulite 1 (samples until July 2011), DPC Immulite 1000 (samples from August 2011 to September 2016) or DPC Immulite 2000 (samples from September 2016 until June 2021); Siemens Schweiz AG, Zurich, Switzerland) validated for cats. 17 Plasma was stored at −20°C until assayed. Serum sodium and potassium concentrations were measured with a Cobas c501 chemistry analyzer (Roche Pharma Schweiz AG, Reinach, Switzerland).
2.3. Ultrasonographic examination of the adrenal glands
Ultrasonography was performed either of a board‐certified radiologist or by a resident under supervision of a board‐certified radiologist after the cats had been stabilized. Ultrasonographic examination was performed in lateral recumbency, and adrenal glands were assessed during the routine abdominal examination. The maximum width was defined as the greatest dorsoventral dimension and was assessed as a single measurement made perpendicular to the long axis.
2.4. Treatment
Mineralocorticoid replacement treatment was started with fludrocortisone (Florinef; Bristol‐Myers Squibb SA, Baar, Switzerland) or DOCP (Zycortal; Dechra Pharmaceuticals, Overland Park, Kansas; or Percorten‐V; Novartis Animal Health US, Greensboro, North Carolina) after an individualized stabilization period including intravenous administration of fluids, treatment of hyperkalemia with glucose infusion and prednisolone administration.
Additional symptomatic treatments and diagnostic testing during hospitalization were at the discretion of the attending clinician.
The starting dose of DOCP was at the discretion of the attending clinician, usually between 1.5 and 2.5 mg/kg SC and the target injection interval was set at q28 to q30 days. The efficacy of DOCP treatment was assessed on day 14 and day 28 after the first injection by monitoring clinical signs and serum electrolyte concentrations (potassium and sodium). Depending on the 14‐ and 28‐day serum potassium and sodium concentrations, the DOCP dosage was adjusted to achieve electrolyte concentrations within the reference interval and a final injection interval of no more than 28 to 30 days.
All cats were treated with prednisolone; starting doses in the cats with newly diagnosed PH ranged between 0.5 and 1 mg/kg IV q6 to q12 hours for a duration of 12 to 48 hours, depending on the severity of the signs and the clinical condition of the cat. Prednisolone treatment was changed to PO as soon as the cats started eating and vomiting stopped (using the same dose which was given IV at that time). At the time of discharge, the prednisolone dosage was decreased (exact numbers are presented in the result section), and further reduction steps were individualized at each recheck based on clinical signs (eg, appetite, activity level, weight gain, polyuria, polydipsia) and on the assessment of the clinician.
Rechecks to adapt the DOCP and the prednisolone doses were usually planned on day 14 and 28 after the first and second DOCP injections and on day 28 after the third injection. Thereafter the rechecks intervals were individualized for each cat.
2.5. Statistical analyses
Statistical analysis was performed by means of nonparametric tests by commercial software (GraphPad Prism 9, GraphPad Software, San Diego, California). Data are expressed as range and median. The change in DOCP dose, prednisolone dose, and electrolyte concentrations between diagnosis and the end of the follow‐up period were evaluated by Wilcoxon matched‐pairs signed rank test. The level of significance was set at P < .05.
3. RESULTS
3.1. Signalment and clinicopathological findings at diagnosis
A total of 11 cats fulfilled the inclusion criteria of the first part of the case series. There were 6 British Shorthair, 4 European Shorthair, and 1 Bengal cats. Seven cats were male (all castrated) and 4 female (3 spayed). Age ranged from 2 to 10 years (median 6.5).
Reported clinical signs by owners included: reduced appetite or anorexia (n = 11), lethargy (10), obstipation (7), weakness (7), loss of body weight (6), vomiting (4), polyuria (4), polydipsia (3), diarrhea (1), tachypnea (1), and empty swallowing (1).
Clinical signs during physical examination included: reduced general condition and lethargy (11), dehydration (9), hypothermia (5), lateral recumbency (4), heart murmur (3), weak pulse (3), acute abdomen (1), tachypnea (1), and seizures (1).
3.2. Routine laboratory findings
The most frequent abnormality found in routine laboratory testing at the time of diagnosis was hyponatremia (11/11 cats, range, 114‐146 mmol/L, median: 135, reference range, 150‐157). Hyperkalemia, increased creatinine, and increased blood urea nitrogen were detected in 8, 8, and 7 cats, respectively (potassium: range, 4.4‐8.1 mmol/L, median: 5.9, reference range, 3.8‐5.4; creatinine: range, 63‐429 μmol/L, median: 225, reference range, 98‐163; blood urea nitrogen: range, 5.2‐32.1 mmol/L, median: 13.9, reference range, 7.4‐12.6).
The results of the routine laboratory testing (including the number of cats with values above or below the reference range) for each laboratory parameter are summarized in Table 1.
TABLE 1.
Findings on routine laboratory examination of 11 cats with primary hypoadrenocorticism.
| Median | Range | No. of cats with value above the reference range | No. of cats with value below the reference range | Reference range | |
|---|---|---|---|---|---|
| Packed cell volume (%) | 38 | 27‐48 | 1/11 | 3/11 | 33‐45 |
| Leucocytes (×10E3/μL) | 9.5 | 5.8‐16.4 | 2/11 | 0/11 | 4.6‐12.8 |
| Thrombocytes (×10E3/μL) | 379 | 87‐591 | 0/6 | 1/6 | 180‐680 |
| Mature neutrophils (×10E3/μL) | 4.85 | 2.88‐11.78 | 1/11 | 0/11 | 2.32‐10.01 |
| Band neutrophils (/μL) | 80 | 50‐690 | 5/11 | NA | 0‐120 |
| Eosinophils (/μL) | 290 | 170‐1150 | 2/9 | 0/9 | 100‐600 |
| Basophils (/μL) | 30 | 0‐140 | 0/7 | 2/7 | 100‐140 |
| Monocytes (/μL) | 110 | 0‐650 | 0/11 | 1/11 | 40‐680 |
| Lymphocytes (/μL) | 2700 | 1250‐5930 | 0/11 | 0/11 | 1050‐6000 |
| Total bilirubin (μmol/L) | 2.5 | 0.8‐3.6 | 1/11 | 0/11 | 0.1‐3.5 |
| Serum glucose (mmol/L) | 7.2 | 4.2‐14.3 | 3/11 | 0/11 | 4‐9 |
| Blood urea nitrogen (mmol/L) | 13.9 | 5.2‐32.1 | 7/11 | 1/11 | 7.4‐12.6 |
| Creatinine (μmol/L) | 225 | 63‐429 | 8/11 | 1/11 | 98‐163 |
| Creatinine kinase (U/L) | 2536 | 193‐23 637 | 8/10 | 0/10 | 77‐355 |
| Protein (g/L) | 63 | 35‐72 | 0/11 | 7/11 | 64‐80 |
| Albumin (g/L) | 32 | 19‐41 | 0/11 | 5/11 | 32‐42 |
| Cholesterol (mmol/L) | 3.7 | 1.1‐4.9 | 0/11 | 3/11 | 2.6‐6.8 |
| Alkaline phosphatase (U/L) | 14.5 | 6‐36 | 0/10 | 6/10 | 16‐43 |
| Alanine aminotransferase (U/L) | 52 | 23‐104 | 1/11 | 1/11 | 34‐98 |
| Aspartate aminotransferase (U/L) | 59 | 19‐220 | 6/11 | 0/11 | 19‐44 |
| DGGR‐lipase (U/L) | 10 | 6‐117 | 1/11 | 0/11 | 6‐26 |
| Sodium (mmol/L) | 135 | 114‐146 | 0/11 | 11/11 | 150‐157 |
| Potassium (mmol/L) | 5.9 | 4.4‐8.1 | 8/11 | 0/11 | 3.8‐5.4 |
| Chloride (mmol/L) | 104 | 83‐116 | 0/11 | 10/11 | 113‐123 |
| Inorganic phosphorus (mmol/L) | 2.0 | 1.2‐4.0 | 8/11 | 0/11 | 0.9‐1.8 |
| Calcium (mmol/L) | 2.5 | 2.04‐3.29 | 1/11 | 4/11 | 2.4‐2.8 |
| Serum cortisol, basal (nmol/L) | <27.6 | <27.6‐69 | 0/11 | 9/11 | <27.6‐257 |
| Serum cortisol, stimulated (nmol/L) | <27.6 | <27.6‐72 | NA | NA | NA |
| Plasma ACTH (pmol/L) | >275 | >275 | 9/9 | 0/9 | 32‐370* |
Abbreviations: ACTH, adrenocorticotropic hormone; DGGR, 1,2‐o‐dilaurylrac‐glycero‐3‐glutaric acid‐(60‐methylresorufin) ester; NA, not applicable.
Reference interval from the literature. 17
3.3. Adrenal gland width on abdominal ultrasound
The width of the left adrenal gland of the 11 cats ranged from 1.2 to 3.9 mm (median: 2 mm) and that of the right adrenal gland from 1.2 to 4.3 mm (median: 2.7). The radiologists judged the adrenal glands of 6 cats to be “smaller than normal.” In these 6 cats, the adrenal gland widths were ≤2 mm on the left side and ≤2.7 mm on the right side (Table S1).
3.4. Type of mineralocorticoid treatment
Ten cats were treated with DOCP: 4 of them were initially treated with Percorten and later administered Zycortal and 6 were treated only with Zycortal. One cat was treated with fludrocortisone (Florinef).
3.5. Hospitalization time
Hospitalization time ranged from 3 to 9 days (median: 4).
3.6. Length of monitoring
Eight cats treated with DOCP were monitored for 14 to 70 months (median: 28 months). Two cats treated with DOCP were euthanized 0.5 and 2 months after discharge. Reasons for euthanasia were financial concerns and problems with giving the prednisolone tablets PO in 1 cat. In this cat, the owner refused to convert the glucocorticoid treatment to a depot preparation. In the other, financial concerns were the main reason, in addition to deterioration and emergency presentation after the general practitioner had not adapted the DOCP dose at the first recheck and the owner had missed the second recheck.
The 1 cat treated with fludrocortisone was lost to follow‐up because the owner refused to treat the cat with fludrocortisone and decided to manage the cat by himself by treating it with prednisolone alone and occasionally hydrocortisone as needed.
3.7. DOCP dosage during long‐term treatment
Of the 8 cats with long‐term follow‐up, 2 were started on DOCP doses ≥2.2 mg/kg (2.2 and 2.5 mg/kg) and 6 on doses <2.2 mg/kg (1.5‐2.0 mg/kg, median 1.8). In both high‐dose cats and 4 of the low‐dose cats the DOCP dosage had to be raised over the first few months of treatment. The maximal DOCP doses used in these 6 cats ranged from 2.3 to 3.0 mg/kg; median: 2.5. The DOCP dosage was decreased in 2 of these cats.
In 2 of the low‐dose cats, the DOCP dose had never been raised during the follow‐up period. In 1 cat (started with 2 mg/kg) the DOCP dosage could be decreased at every recheck to a final dose of 1.3 mg/kg. In the other cat, the DOCP dose was kept unchanged at the dosage of 2 mg/kg for 4 years but thereafter had to be decreased to 1.7 mg/kg. DOCP doses at the end of the follow‐up period of all cats ranged between 1.3 and 3 mg/kg (median: 2.3).
There was no significant difference between the DOCP starting dose and the DOCP dose at the end of the follow‐up period (P = .19). DOCP doses of each cat at both time points are presented in Figure 1.
FIGURE 1.
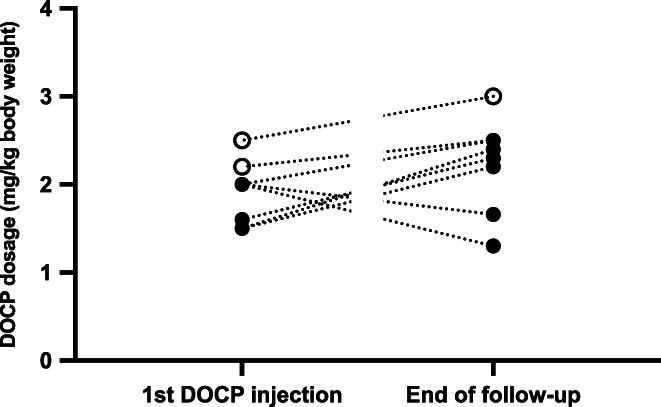
Desoxycorticosterone pivalate dosage (mg/kg body weight) at the time of the first injection and at the end of the follow‐up period of each cat. Open circles represent cats started on a DOCP dosage ≥2.2 mg/kg. Closed circles represent cats started on a DOCP dosage <2.2 mg/kg
3.8. Glucocorticoid treatment during long‐term treatment
All 8 cats were discharged on prednisolone of which the dosage at discharge ranged from 0.4 to 1.0 mg/kg/day (median: 0.73). During the follow‐up period, the prednisolone dose could be further reduced in all cats to a final prednisolone dose of 0.08 to 0.5 mg/kg/day (median: 0.3) at the end of the follow‐up period. There was a significant decrease in the prednisolone dose between discharge and the end of the follow‐up (P = .0078). Prednisolone doses of each cat at both time points are presented in Figure 2.
FIGURE 2.
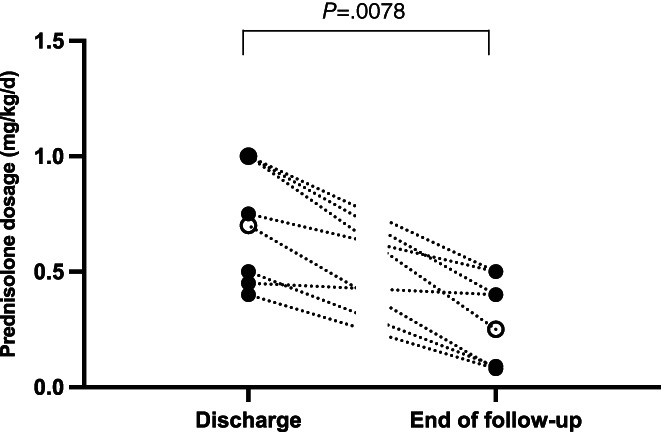
Prednisolone dosage (mg/kg/d) at the time of discharge and at the end of the follow‐up period of each cat. Open circles represent cats started on a DOCP dosage ≥2.2 mg/kg. Closed circles represent cats started on a DOCP dosage <2.2 mg/kg
3.9. Serum potassium and sodium concentrations
Serum potassium concentrations decreased with treatment. The decrease between diagnosis and the last follow‐up was significant (P = .02). Serum sodium concentrations increased with treatment. The increase between diagnosis and the last follow‐up was significant (P = .02). Potassium and sodium concentrations of each cat at both time points are presented in Figure 3.
FIGURE 3.
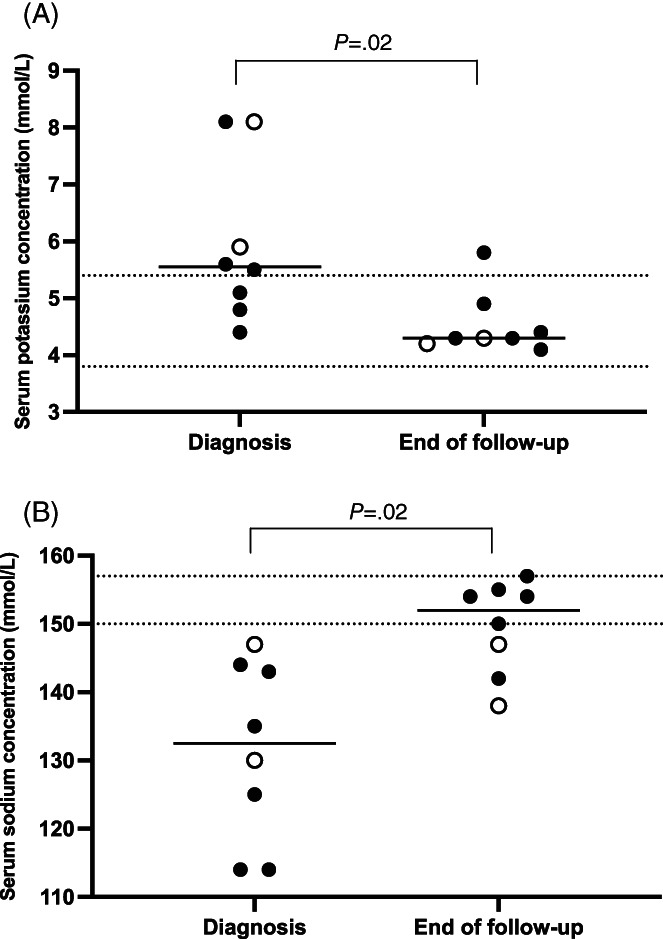
Serum potassium (A) and serum sodium (B) concentrations (mmol/L) at the time of diagnosis and at the end of the follow‐up period of each cat. Open circles represent cats started on a DOCP dosage ≥2.2 mg/kg. Closed circles represent cats started on a DOCP dosage <2.2 mg/kg. The area between the dotted lines represents the reference range of the serum potassium or sodium concentration
4. DISCUSSION
Results of this study provide information about naturally occurring PH in cats with a focus on long‐term treatment with DOCP and prednisolone. This work shows that the starting dose in cats should be 2.2 mg/kg as recommended by the manufacturer. After 12 months of treatment, all but 1 cat still needed a DOCP dose of at least 2 mg/kg q28 days. Further, in 4 of 6 cats that had been started on a low DOCP dose (1.5‐2 mg/kg) and the 2 cats started on DOCP doses ≥2.2 mg/kg, the dose had to be increased within the first few months. There was only 1 cat in which the starting dose of 2 mg/kg was kept constant and 1 cat (started on 2 mg/kg) in which the dose could be decreased within the first months of treatment. This is in complete contrast to what has been shown in dogs: dogs can be stabilized with a much lower DOCP dose or by prolonging the injection interval. 12 , 13 , 14 , 15 , 16 Interestingly, and in contrast to cats, the maintenance dose of DOCP could be further reduced (to a median of 1.1 mg/kg, range, 0.7‐1.8) after several months of treatment in the majority of dogs, without the risk of underdosing, in 1 study. 14 The reason why cats with PH need higher doses of mineralocorticoids is not entirely clear. Cats have approximately half the density of glucocorticoid receptors in their skin and liver compared to dogs, and the receptors have a lower binding affinity than in dogs. 18 , 19 Whether this holds true for the mineralocorticoid receptor is not known, but it would be a reasonable explanation for why cats need higher DOCP doses than dogs.
The final long‐term prednisolone dose cats needed in this study was around 0.3 mg/kg/day, which is higher than the commonly recommended dose of about 0.05 to 0.1 mg/kg/day in dogs. As discussed above, the most likely explanation for the higher glucocorticoid dose needed in cats is the reduced density of glucocorticoid receptors in tissue and the lower binding affinity of glucocorticoids at the receptor. 18 , 19 However, that some of these cats were overtreated with prednisolone cannot be completely excluded. The prednisolone dose adjustment during long‐term treatment is difficult. Most often, adjustments are based only on the clinical impression of the owner and the veterinarian. In cats, clinical signs of prednisolone overtreatment are challenging to detect, because cats usually do not show increased thirst and may only show increased sleeping and gain in body weight. In dogs, the integration of endogenous ACTH measurements into the treatment surveillance has been suggested by some authors. 20 Endogenous ACTH seemed especially helpful in dogs with ambiguous clinical signs, and a completely suppressed ACTH concentration during a hospital visit was interpreted as a likely signal of overtreatment. 20 Endogenous ACTH concentrations were not assessed during follow‐up in this study. In future, however, endogenous ACTH concentrations should be evaluated to explore their usefulness in guiding long‐term glucocorticoid treatment in cats.
Two cats were euthanized within the first 2 months after diagnosis. In both cats, financial concerns were 1 of the main reasons for euthanasia. As is well known from dogs, the costs of mineralocorticoid medication are a treatment‐limiting factor for some owners of animals with PH. This is the reason why many endocrinologists have started to use lower DOCP doses or increase the interval between 2 DOCP injections. 12 , 13 , 14 , 15 , 16 Both methods will decrease the cost of treatment. In cats, however, as discussed above, decreasing the DOCP dose does not seem a safe option. Whether some cats could tolerate a prolonged dosing interval cannot be answered here, because it was not the goal of the study.
Potassium concentrations decreased and sodium concentrations increased during treatment. However, a comparison of the electrolyte trends in cats and dogs with PH that have been treated with DOCP revealed that sodium concentrations in particular are more widely distributed and more often below the reference range in cats. 14 In the authors' laboratory, the reference interval for sodium in cats (150‐157 mmol/L) is higher than that of dogs (145‐152 mmol/L). However, because the measured values are so variably distributed in cats, this does not seem to be a likely explanation. Rather, the often‐decreased sodium concentrations are a possible sign of insufficient mineralocorticoid treatment in these cats. In human medicine, plasma renin activity is the most sensitive marker for identifying insufficient or excessive mineralocorticoid replacement. 21 , 22 In dogs, plasma renin activity decreased and normalized with DOCP treatment, but not with fludrocortisone treatment. 23 Fludrocortisone treatment was therefore considered less effective in dogs, which was in line with the frequently decreased sodium concentration in dogs on fludrocortisone. 23 An insufficient mineralocorticoid replacement treatment could possibly even influence the prednisolone need of cats. Prednisolone is known to have some mild mineralocorticoid effects. 24 Therefore, insufficient mineralocorticoid replacement could, at least partially, be cushioned by an increased prednisolone dose. Plasma renin activities were not assessed in the cats of this study. Future studies in cats with PH should, however, evaluate renin activity to estimate the effectiveness of the DOCP treatment in this species.
All cats of this study with PH were adults, with 8 of 11 aged >5 years. Although many cats with hypoadrenocorticism have been reported to be younger than 5 years, it is known that the disease can occur in a broad age range. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 A possible explanation for the higher age of the cats in this study, could be the high number of pure breed cats (7/11), which are more often indoor cats, and therefore signs like reduced activity and lethargy might only be detected by owners late in the course of the disease. In dogs, it has been shown that younger dogs with PH (≤3 years) need significantly higher doses of DOCP than older dogs. 14 Because only 2 of the cats with long‐term follow‐up were <3 years and the total number of cases is low, a possible age factor cannot be evaluated in this case series. The median age of cats needing DOCP doses >2.2 mg/kg or <2.2 mg/kg at the end of the follow‐up period was however similar (6.5 vs 6.4 years).
Six of 11 cats were British Shorthair. This is a new and interesting finding because, so far, no breed predispositions have been reported in cats. In dogs, it is well known that breed predispositions exist, and inheritance has been proven for some breeds. 25 , 26 , 27 , 28 , 29 Further studies in British shorthair cats are therefore warranted to evaluate a possible heritability of PH in this breed.
The signs reported by the owner and clinical signs noted during initial examination of cats with PH in this study were similar to those of cats described in earlier case reports and case series and were mainly unspecific. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Interestingly, obstipation was a very common sign (7/11) in this case series. In the 18 cases of PH in cats reported in the literature, obstipation or constipation was only mentioned in 2 cats. 3 , 5 That PH could lead to obstipation in cats seems reasonable, because many of these patients have been sick for several days and are often severely dehydrated. Interestingly, obstipation is not a typical sign expected in dogs with PH either. Dogs more often have diarrhea, which is an uncommon sign in cats.
The absence of a stress leukogram in an ill (stressed) animal is often discussed as a classical hematologic finding in individuals with PH. Sometimes even lymphocytosis and eosinophilia can be seen in these patients. 2 All the cats in this study had normal lymphocyte counts, 2 displayed eosinophilia, and 1 neutrophilia. Quiet typically, most cats were azotemic and many had low total protein and low albumin concentrations. Three cats were hyperglycemic at the time of diagnosis. Pretreatment with glucose solutions by the RDVM or during emergency stabilization at the clinic could be excluded in at least 2 cases and was considered highly unlikely in 1 cat. The presence of hyperglycemia in cats is different from dogs, in which hypoglycemia occurs more often. Hypoglycemia in dogs is assumed to be because of decreased hepatic gluconeogenesis and increased peripheral sensitivity to insulin. 30 , 31 Why cats are more likely to exhibit hyperglycemia is unclear. However, it may be related to the more frequent occurrence of stress hyperglycemia in cats, for which the mechanisms are still not entirely clear. 32 Finally, the majority of cats had creatinine kinase (CK) concentrations above the reference range and in some cases, the values were markedly increased. Elevated CK concentrations have been described in cats with PH and are often a sign for recent muscle lesions. 4 Intramuscular drug administration can lead to mild to moderate elevations, severe elevations are most often observed after a seizure episode. In this study, 1 cat was admitted with seizures. However, we cannot exclude that some of the other cats with severely increased CK had unrecognized episodes of seizures before the cats had been presented.
All cats in this case series received an abdominal ultrasound examination with evaluation of the adrenal glands during the regular workup. In 6 of the 11 cats, the radiologists judged the width of the adrenal glands to be smaller than normal. Several studies report adrenal gland sizes of healthy cats, but there is no concordance and therefore not a single reference interval. 11 , 33 , 34 , 35 , 36 , 37 Adrenal gland sizes in cats with PH are only rarely reported, however, where descriptions are available, the adrenal glands have been reported to be normal in size. 5 , 6 , 7 , 8 This is striking because this is contrary to reports on dogs, in which small adrenal glands have been reported. 10 So far, there seems only 1 report of a cat with PH with small adrenal glands. 11 In this study, we describe 6 of 11 cats with PH with small adrenal glands. Importantly, however, normal‐sized adrenal glands in cats with suspected PH do not exclude the disease.
A number of limitations to this case series must be acknowledged: First, the number of cats included is low. However, although the number is low, this case series is still the largest to be reported so far, which underlines the rarity of this disease in cats. Second, the ultrasonographic examination of the adrenal glands was not standardized and was not performed by the same radiologist. However, all adrenal measurements were performed either by a board‐certified radiologist or under the supervision of a board‐certified radiologist. Thus, the case series includes the largest number of cats with PH and also the largest number for which adrenal gland sizes are reported. Another potential limitation is that the starting dose of DOCP and its reduction scheme was not standardized but was left to the discretion of the attending clinician. Finally, the dose reduction scheme of prednisolone was also not standardized for cats. A standardized protocol for dogs exists in the author's clinic which most clinicians also use in an adapted form for cats. The last 2 points could also have influenced the results of this work.
In conclusion, the need of DOCP in cats with PH seems to be higher than in dogs. Therefore, a starting dose of 2.2 mg/kg as recommended by the manufacturer for dogs, seems warranted in cats. Dose adjustment during long‐term treatment should then be performed in the same way as for dogs. In addition, the need for administration of prednisolone seems higher in cats as well. As a rough guideline, a long‐term prednisolone dose of 0.3 mg/kg/day can be aimed at, which is then titrated to individual needs. British Shorthair cats seemed overrepresented in this case series. This is the first time that a possible breed predisposition for PH in cats has been demonstrated. Further studies are needed to evaluate the genetic background of this disease in cats and evaluate whether this phenomenon is more of a local or a global problem. Even though no accepted reference range exists for the adrenal gland sizes of healthy cats, cats with PH can have very small adrenal gland widths. Therefore, in a cat with unspecific clinical signs and no reported history of glucocorticoid treatment, adrenal gland widths of <2.7 mm should be interpreted as an indication to investigate the cat for PH.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
TABLE S1. Width of the left and right adrenal glands of the 11 cats with PH
ACKNOWLEDGMENT
No funding was received for this study. The authors gratefully acknowledge the veterinarians of the Clinic for Small Animal Internal Medicine for their contribution of cases.
Sieber‐Ruckstuhl NS, Harburger L, Hofer N, et al. Clinical features and long‐term management of cats with primary hypoadrenocorticism using desoxycorticosterone pivalate and prednisolone. J Vet Intern Med. 2023;37(2):420‐427. doi: 10.1111/jvim.16658
The work was performed at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty, University of Zurich, Switzerland.
REFERENCES
- 1. Bell R, Mellor DJ, Ramsey I, et al. Decreased sodium:potassium ratios in cats: 49 cases. Vet Clin Pathol. 2005;34:110‐114. [DOI] [PubMed] [Google Scholar]
- 2. Peterson ME, Greco DS, Orth DN. Primary hypoadrenocorticism in ten cats. J Vet Intern Med. 1989;3:55‐58. [DOI] [PubMed] [Google Scholar]
- 3. Tasker S, MacKay AD, Sparkes AH. A case of feline primary hypoadrenocorticism. J Feline Med Surg. 1999;1:257‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stonehewer J, Tasker S. Hypoadrenocorticism in a cat. J Small Anim Pract. 2001;42:186‐190. [DOI] [PubMed] [Google Scholar]
- 5. Kasabalis D, Bodina E, Saridomichelakis MN. Severe Hypoglycaemia in a cat with primary hypoadrenocorticism. J Feline Med Surg. 2012;14:755‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sicken J, Neiger R. Addisonian crisis and severe acidosis in a cat: a case of feline hypoadrenocorticism. J Feline Med Surg. 2013;15:941‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Woolcock AD, Ward C. Successful treatment of a cat with primary hypoadrenocorticism and severe hyponatremia with desoxycorticosterone pivalate (DOCP). Can Vet J. 2015;56:1158‐1160. [PMC free article] [PubMed] [Google Scholar]
- 8. Fowlie SJ, McKenzie J, Ramsey I. Hypoadrenocorticism in an aged cat. Vet Rec Case Rep. 2018;6:e000565. [Google Scholar]
- 9. Reiman F, Siol S, Schlüter C, et al. Hypoadrenocorticism in two cats successfully treated with desoxycorticosterone pivalate. Vet Rec Case Rep. 2018;6:e000600. [Google Scholar]
- 10. Wenger M, Mueller C, Kook PH, Reusch CE. Ultrasonographic evaluation of adrenal glands in dogs with primary hypoadrenocorticism or mimicking diseases. Vet Rec. 2010;167:207‐210. [DOI] [PubMed] [Google Scholar]
- 11. Griffin S. Feline abdominal ultrasonography: What's normal? What's abnormal? The adrenal glands. J Feline Med Surg. 2021;23:33‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bates JA, Shott S, Schall WD. Lower initial dose desoxycorticosterone pivalate for treatment of canine primary hypoadrenocorticism. Aust Vet J. 2013;91:77‐82. [DOI] [PubMed] [Google Scholar]
- 13. Jaffey JA, Nurre P, Cannon AB, DeClue AE. Desoxycorticosterone Pivalate duration of action and individualized dosing intervals in dogs with primary Hypoadrenocorticism. J Vet Intern Med. 2017;31:1649‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sieber‐Ruckstuhl NS, Reusch CE, Hofer‐Inteeworn N, et al. Evaluation of a low‐dose desoxycorticosterone pivalate treatment protocol for long‐term management of dogs with primary hypoadrenocorticism. J Vet Intern Med. 2019;33:1266‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Münch L, Münch M, Paul H, Miklis A, Heinrich M, Neiger R. Therapie des primären Hypoadrenokortizismus beim Hund mit niedrig dosiertem desoxycorticosteronpivalat [therapy of primary hypoadrenocorticism in dogs with low dose desoxycorticosterone pivalate]. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2020;48:171‐175. [DOI] [PubMed] [Google Scholar]
- 16. Vincent AM, Okonkowski LK, Brudvig JM, et al. Low‐dose desoxycorticosterone pivalate treatment of hypoadrenocorticism in dogs: a randomized controlled clinical trial. J Vet Intern Med. 2021;35:1720‐1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tardo AM, Reusch CE, Galac S, et al. Feline plasma adrenocorticotropic hormone: validation of a chemiluminescent assay and concentrations in cats with hypercortisolism, primary hypoadrenocorticism and other diseases. J Feline Med Surg. 2021;23:67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van den Broek AH, Stafford WL. Epidermal and hepatic glucocorticoid receptors in cats and dogs. Res Vet Sci. 1992;52:312‐315. [DOI] [PubMed] [Google Scholar]
- 19. Lowe AD, Campbell KL, Graves T. Glucocorticoids in the cat. Vet Dermatol. 2008;19:340‐347. [DOI] [PubMed] [Google Scholar]
- 20. Zeugswetter FK, Haninger T. Prednisolone dosages in Addisonian dogs after integration of ACTH measurement into treatment surveillance. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2018;46:90‐96. [DOI] [PubMed] [Google Scholar]
- 21. Oelkers W, Diederich S, Bähr V. Diagnosis and therapy surveillance in Addison's disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992;75:259‐264. [DOI] [PubMed] [Google Scholar]
- 22. Reisch N, Arlt W. Fine tuning for quality of life: 21st century approach to treatment of Addison's disease. Endocrinol Metab Clin North Am. 2009;38:407‐418. [DOI] [PubMed] [Google Scholar]
- 23. Baumstark ME, Nussberger J, Boretti FS, et al. Use of plasma renin activity to monitor mineralocorticoid treatment in dogs with primary hypoadrenocorticism: desoxycorticosterone versus fludrocortisone. J Vet Intern Med. 2014;28:1471‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pearn JH. Use of corticosteroids in childhood disease. Drugs. 1975;10:426‐436. [DOI] [PubMed] [Google Scholar]
- 25. Oberbauer AM, Benemann KS, Belanger JM, Wagner DR, Ward JH, Famula TR. Inheritance of hypoadrenocorticism in bearded collies. Am J Vet Res. 2002;63:643‐647. [DOI] [PubMed] [Google Scholar]
- 26. Famula TR, Belanger JM, Oberbauer AM. Heritability and complex segregation analysis of hypoadrenocorticism in the standard poodle. J Small Anim Pract. 2003;44:8‐12. [DOI] [PubMed] [Google Scholar]
- 27. Oberbauer AM, Bell JS, Belanger JM, Famula TR. Genetic evaluation of Addison's disease in the Portuguese water dog. BMC Vet Res. 2006;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hughes AM, Nelson RW, Famula TR, Bannasch DL. Clinical features and heritability of hypoadrenocorticism in Nova Scotia duck tolling retrievers: 25 cases (1994‐2006). J Am Vet Med Assoc. 2007;231:407‐412. [DOI] [PubMed] [Google Scholar]
- 29. Hughes AM, Bannasch DL, Kellett K, Oberbauer AM. Examination of candidate genes for hypoadrenocorticism in Nova Scotia duck tolling retrievers. Vet J. 2011;187:212‐216. [DOI] [PubMed] [Google Scholar]
- 30. Lifton SJ, King LG, Zerbe CA. Glucocorticoid deficient hypoadrenocorticism in dogs: 18 cases (1986‐1995). J Am Vet Med Assoc. 1996;209:2076‐2081. [PubMed] [Google Scholar]
- 31. Gow AG, Gow DJ, Bell R, Evans H, Mellanby RJ. Insulin concentrations in dogs with hypoadrenocorticism. Res Vet Sci. 2012;93:97‐99. [DOI] [PubMed] [Google Scholar]
- 32. Rand JS, Kinnaird E, Baglioni A, Blackshaw J, Priest J. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med. 2002;16:123‐132. [DOI] [PubMed] [Google Scholar]
- 33. Cartee RE, Bodner STF, Gray BW. Ultrasound examination of the feline adrenal gland. J Diag Med Sonog. 1993;9:327‐330. [Google Scholar]
- 34. Zimmer C, Hörauf A, Reusch C. Ultrasonographic examination of the adrenal gland and evaluation of the hypophyseal‐adrenal axis in 20 cats. J Small Anim Pract. 2000;41:156‐160. [DOI] [PubMed] [Google Scholar]
- 35. Zatelli A, D'Ippolito P, Fiore I, et al. Ultrasonographic evaluation of the size of the adrenal glands of 24 diseased cats without endocrinopathies. Vet Rec. 2007;160:658‐660. [DOI] [PubMed] [Google Scholar]
- 36. Combes A, Vandermeulen E, Duchateau L, Peremans K, Daminet S, Saunders J. Ultrasonographic measurements of adrenal glands in cats with hyperthyroidism. Vet Radiol Ultrasound. 2012;53:210‐216. [DOI] [PubMed] [Google Scholar]
- 37. Combes A, Pey P, Paepe D, et al. Ultrasonographic appearance of adrenal glands in healthy and sick cats. J Feline Med Surg. 2013;15:445‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Width of the left and right adrenal glands of the 11 cats with PH


