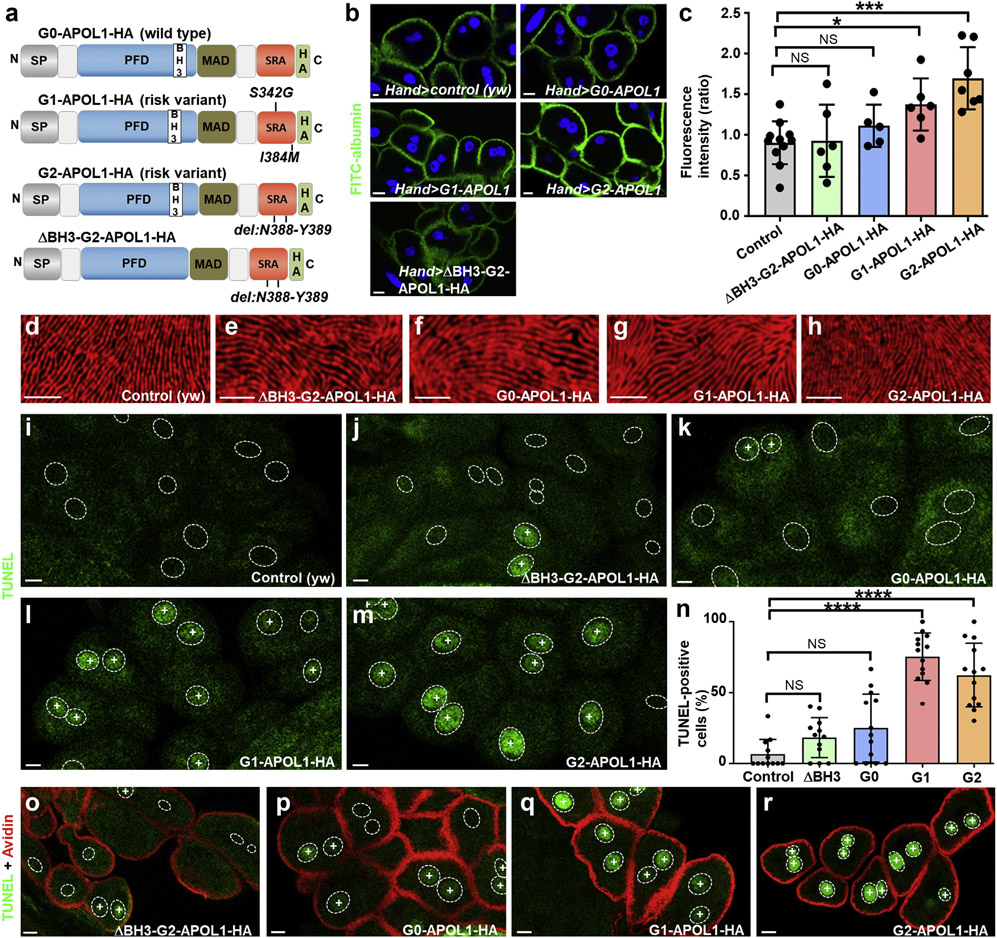
Figure 1 ∣. APOL1 risk variants increase nephrocyte function and significantly increase the fraction of cells positive for the cell death marker terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling (TUNEL).
(a) Schematic indicating transgenic APOL1 constructs employed in this study. The functional protein domains of APOL1 encompass an N-terminal signal peptide that is followed by the pore forming domain (PFD), which harbors a BCL2 homology domain 3 (BH3). The PFD is followed by the membrane addressing (MAD) and finally the serum resistance antigen interacting domains (SRAs) before the C-terminus. All constructs carry an influenza hemagglutinin (HA) tag at the C-terminus unless otherwise indicated. G0-APOL1 represents a wild-type APOL1 sequence (top), whereas G1-APOL1 carries 2 missense variants (p.S342G and p.I384M) and G2-APOL1 a deletion of 2 amino acids (del: N388/Y389). ΔBH3-G2-APOL1 (bottom) carries an additional short deletion (del: L158-Q166) within the BH3 domain and is otherwise identical with G2-APOL1. Deletion of these residues has been shown to abrogate toxicity in cultured human cells,25 so we chose this as a control variant. (b) Expression of APOL1 risk variants G1-APOL1 and G2-APOL1, but not expression of ΔBH3-G2-APOL1 and G0-APOL1 in Drosophila garland cell nephrocytes using Hand-GAL4 increases uptake of fluorescein isothiocyanate–albumin as an established assay of nephrocyte function. Flies were raised at 29 °C. Nuclei are marked by Hoechst 33342 in blue here and throughout the figure. (c) Quantitation of data in (b); mean fluorescence per animal based on the 3 brightest cells in ratio to control experiment is shown; n = 6–11 animals per genotype; P < 0.05 for G1-APOL1 and P < 0.001 for G2-APOL1). (d–h) Tangential sections of control garland cell nephrocytes (d) or garland cell nephrocytes expressing ΔBH3-G2-APOL1 (e), G0-APOL1 (f), G1-APOL1 (g), or G2-APOL1 (h), using Hand-GAL4 at 29 °C are shown. Cells are stained for KIRREL/NEPH1 ortholog Kirre (red), revealing an unaffected staining pattern of slit diaphragms for all indicated genotypes. (b, i–m, o–r) Bar = 10 μm. (d–h) Bar = 5 μm. (i–m) TUNEL labeling of garland cell nephrocytes from third instar larvae is shown for the indicated genotypes, and positivity is indicated by an intranuclear “+.” Outlines of nuclei are shown by dotted circles based on Hoechst 33342 staining (data not shown). (i) In control animals (dKlf15-GAL4/+), TUNEL-positive nephrocytes are rare or absent. TUNEL-positive cells become slightly more frequent with expression of control protein ΔBH3-G2-APOL1 (j) and wild-type G0-APOL1 (k). In contrast, the risk variants G1-APOL1 (l) and G2-APOL1 (m) caused a high fraction of TUNEL-positive cells. (n) Quantitation of data in (i–m); the fraction of TUNEL-positive cells is shown (percentage); n = 12 to 15 animals per genotype; P > 0.05 for ΔBH3-G2-APOL1 and G0-APOL1, and P < 0.001 for G1- and G2-APOL1 compared with control animals. (o–r) Shown are nephrocytes that were exposed to red fluorescent tracer Texas Red–avidin as a readout of nephrocyte function followed by TUNEL staining. TUNEL-positive nephrocytes show an equal uptake compared with TUNEL-negative neighboring cells in ΔBH3-G2-APOL1 (o) and G0-APOL1 (p) expressing cells. Increased endocytosis of Texas Red–avidin is observed in G1-APOL1 (q) and G2-APOL1 (r) expressing nephrocytes despite widespread TUNEL positivity. NS, not significant. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.