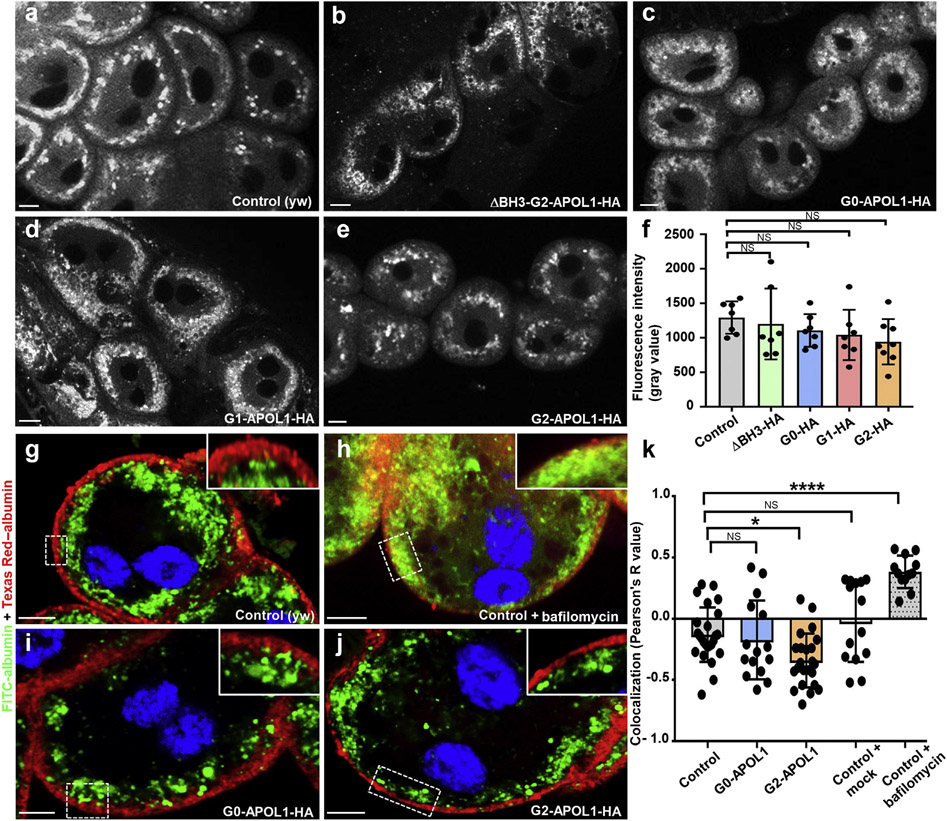
Figure 2 ∣. Expression of APOL1 risk variants in garland cell nephrocytes does not reduce lysotracker staining and induces no overt defect in endocytosis.
(a–e) Confocal live cell imaging of garland cell nephrocytes dissected from third instar larvae reveals acidic vesicles because the living cells were exposed to Lysotracker Red DND-99 at 0.25 μM for 5 minutes before immediate recording. Fluorescence intensity of the indicated genotypes appears comparable. (f) Quantitation of dye-derived fluorescence intensity from data analogous to (a–e), expressed as mean fluorescence intensity per animal, indicates no significant difference compared with control animals (Hand-GAL/+) for any APOL1 variant studied here (n = 7–8; P > 0.05 for all indicated genotypes). (g–j) Shown are nephrocytes that were exposed to 2 consecutive tracers ex vivo. First fluorescein isothiocyanate (FITC)–albumin (green) followed by chase period and finally Texas Red–albumin (red). Note how both tracers become spatially separated through endocytic progressing and progression toward late endosomes in control cells (Hand-GAL4/+) (g), whereas a block of endolysosomal acidification results in a partial colocalization (h). In contrast, expression of G0-APOL1 (i) and G2-APOL1 (j) did not induce colocalization, suggesting a largely unimpaired processing of endocytic cargo. Flies were raised at 29 °C. (k) Quantitation of data analogous to (g–j) using Pearson’s coefficient (3 individual cells each from 4–8 animals are shown per genotype; P < 0.05 for G2-APOL1 and P < 0.0001 for bafilomycin treatment). Pearson’s coefficient ranges from +1 (perfect colocalization) to −1 (perfect separation). With G2-APOL1, the separation becomes even more pronounced, suggesting swifter endocytic processing. Bafilomycin is the only condition that delays endocytic processing. NS, not significant. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.