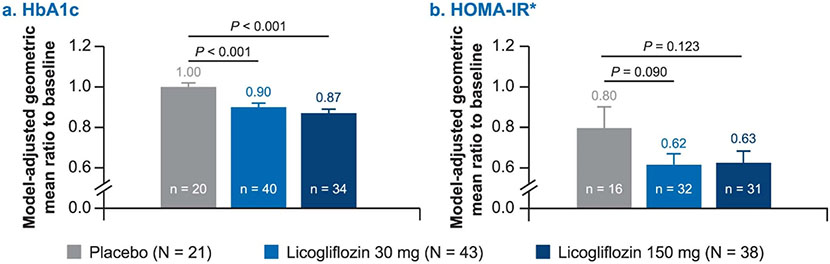
Extended Data Fig. 6: Change in metabolic parameters from baseline up to week 12.
a, HbA1c; b, HOMA-IR. *Ten patients who received exogenous insulin were excluded from HOMA-IR calculation (placebo (n = 3), licogliflozin 30 mg (n = 5), and licogliflozin 150 mg (n = 2)). Data presented as geometric mean ratio to baseline. P values based on two-sided ANCOVA test. Baseline is defined as the last available measurement prior to the first dose. n, number of patients in each treatment group. N for placebo, licogliflozin 30 mg, and licogliflozin 150 mg for HbA1c are 20, 40, and 34; N for HOMA-IR are placebo (n = 16), licogliflozin 30 mg (n = 32), and licogliflozin 150 mg (n = 31). Error bars represent s.e.