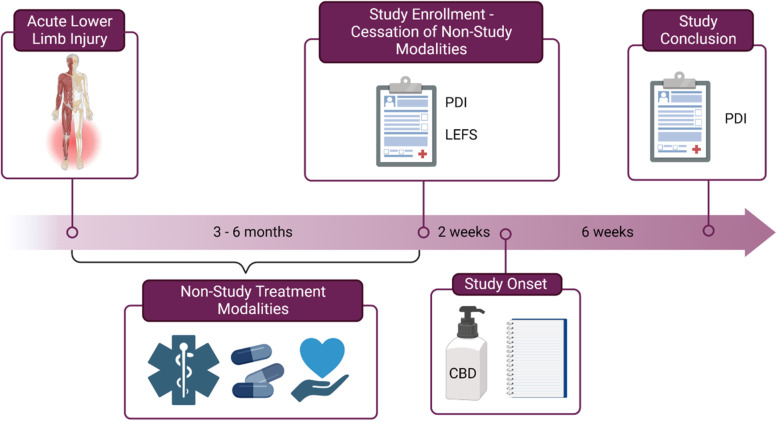
Fig. 1.
Timeline of the study design. The participants reported experiencing a lower extremity injury 3–6 months prior to enrollment. They then underwent various non-study related therapeutic modalities, which they self-reported to be ineffective. At study enrollment, they were asked to discontinue all of these modalities during the study period. At intake, participants completed the Pain Disability Index (PDI) and the Lower Extremity Functional Scale (LEFS). They also began to complete a daily pain journal. Participants received cannabidiol (CBD) treatment at 10 mg twice daily by controlled topical dispenser for six weeks. At the conclusion of the study, they completed their last entry in the pain journal as well as a second PDI