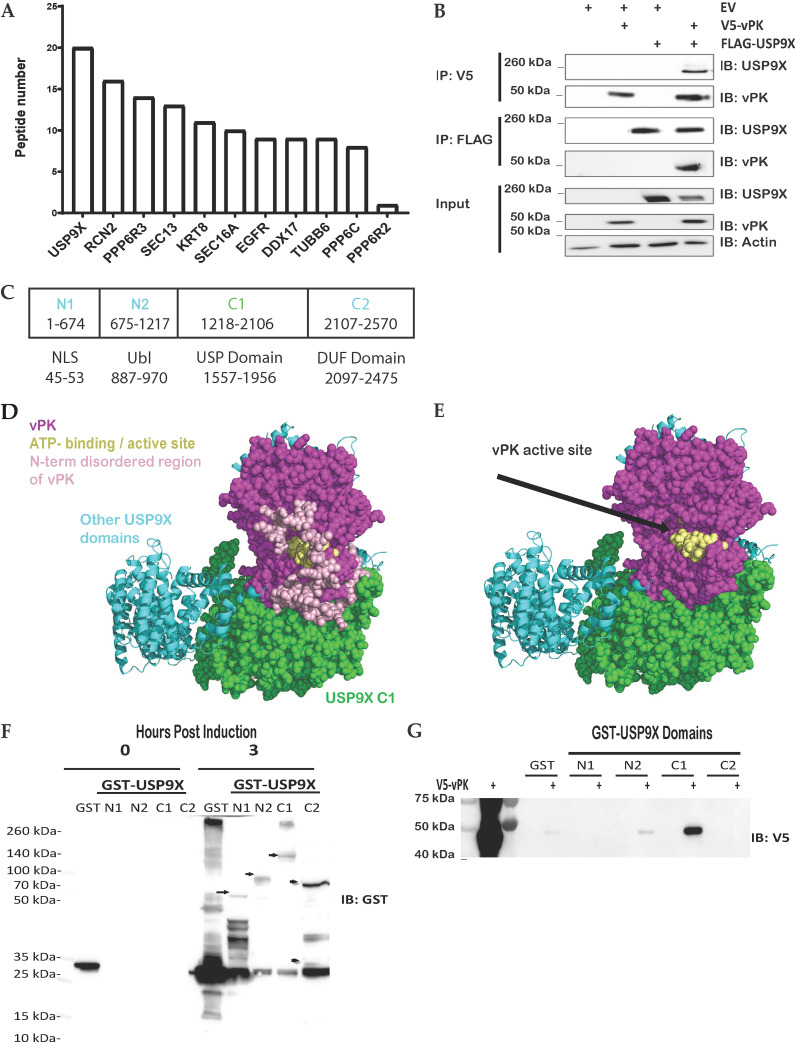
FIG 1.
vPK interacts with USP9X. (A) Either FLAG-tagged GFP or FLAG-tagged vPK expressing HEK293 cell lysates were subjected to mass spectrometry after immunoprecipitation with an anti-FLAG antibody to pull down proteins interacting with vPK. Peptides shown are positive only for FLAG-vPK pull-down and were negative for FLAG-GFP pull down. (B) FLAG-tagged USP9X and V5-tagged vPK were transfected either individually or together into HEK293T cells for 72 h. Cell lysates were immunoprecipitated with anti-FLAG or anti-V5 antibodies and then immunoblotted with the indicated antibodies. (C) Schematic of the domains and truncated regions (N1, N2, C1, C2) of USP9X which includes a nuclear localization signal (NLS), a ubiquitin-like (Ubl) domain, a ubiquitin-specific protease (USP), and a domain of unknown function (DUF). (D) The AlphaFold prediction docking pose of vPK (magenta, and yellow spheres for the ATP binding site) places vPK near the C1 domain (green spheres) of USP9X. (E) The AlphaFold model of the C1 domain of USP9X was the same as in D except for the modeled disordered loops from N1. (F) Plasmids expressing GST alone (GST) and the indicated GST-USP9X truncation mutants were expressed in BL21Ai competent cells. Protein expression was induced using L-arabinose or IPTG (GST control) and purified via GST pulldown. Arrows show the size of the USP9X truncation mutants. (G) GST alone (GST) and the indicated GST-USP9X truncation mutants from panel F were incubated with equal amounts of lysates from 293T cells expressing V5-tagged vPK. After GST pulldown assays, the amount of bound V5-tagged vPK was analyzed by Western blotting. These experiments were performed with at least three biological replicates.