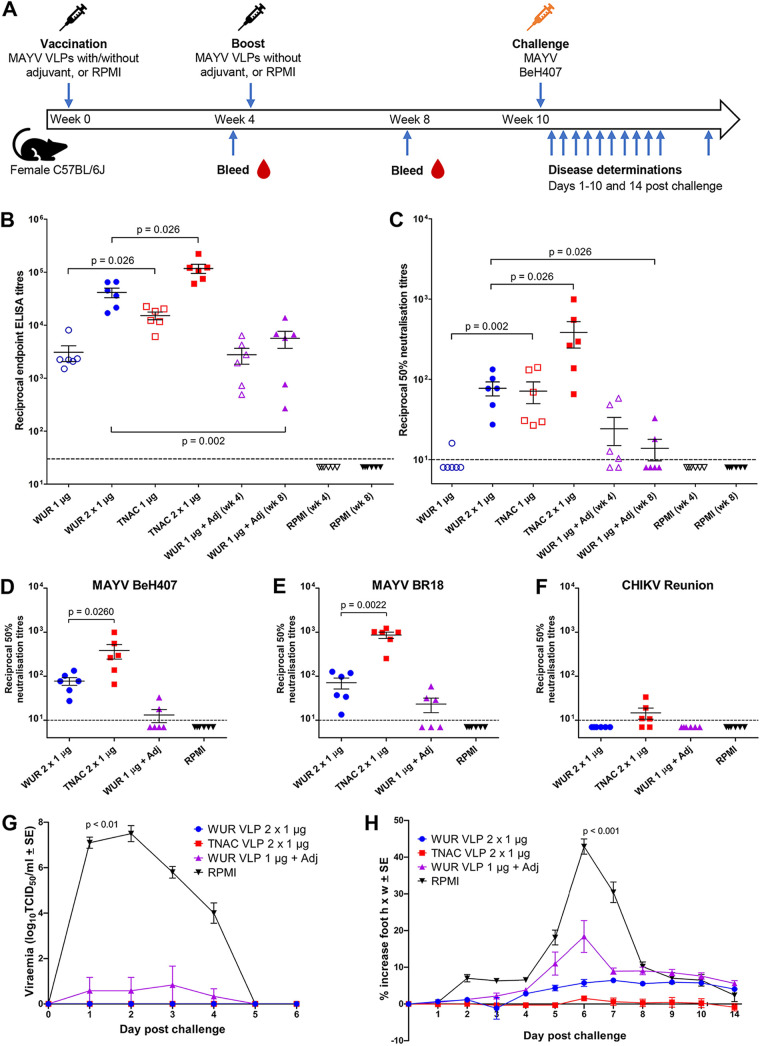
FIG 3.
MAYV VLP vaccination and challenge with MAYV BeH407 in adult C57BL/6J mice. (A) Timeline of vaccination with two 1-μg doses of nonadjuvanted MAYV VLPs, with a single 1-μg dose of adjuvanted MAYV VLPs, or with two doses of RPMI 1640 medium (negative control), then antibody measurements after bleeds, and disease determinations of viremia and foot swelling following MAYV BeH407 challenge. (B) MAYV BeH407 endpoint IgG ELISA titers after 1 or 2 vaccinations of female 6 -to 8-week-old C57BL/6J mice with nonadjuvanted MAYV VLPs or RPMI control, or 1 vaccination with MAYV VLPs with adjuvant. Lines among the dots indicate the mean ELISA titers, and error bars show the standard errors of the means. Dashed line represents the limit of detection (1:30 serum dilution). Statistical analysis used the Kolmogorov-Smirnov test. Multiple test correction was not applied. (C) MAYV BeH407 50% neutralization titers after 1 or 2 vaccinations with nonadjuvanted MAYV VLPs or RPMI control, or 1 vaccination with MAYV VLPs with adjuvant. Lines among the dots indicate the mean neutralization titers, and error bars show the standard errors of the means. Dashed line represents the limit of detection (1:10 serum dilution). Statistical analysis was with the Kolmogorov-Smirnov test. Multiple test correction was not applied. (D to F) Comparison of neutralization titers at week 8 against MAYV BeH407 (D), MAYV BR-18 (E), and CHIKV (Reunion isolate) (F). (G) MAYV BeH407 viremia postchallenge in mice vaccinated twice with nonadjuvanted MAYV VLPs or RPMI, or vaccinated once with MAYV VLPs with adjuvant (n = 5 to 6 per group). The limit of detection for each mouse was 102 TCID50/mL, with means from 5/6 mice plotted. Statistical analysis was with the Kolmogorov-Smirnov test. Multiple test correction was not applied. (H) Percentage increase in foot height × width (relative to day 0) for C57BL/6J mice vaccinated as described for panel G, with n = 6 to 12 feet from 3 to 6 mice per group per time point. Statistical analysis was with the t test.