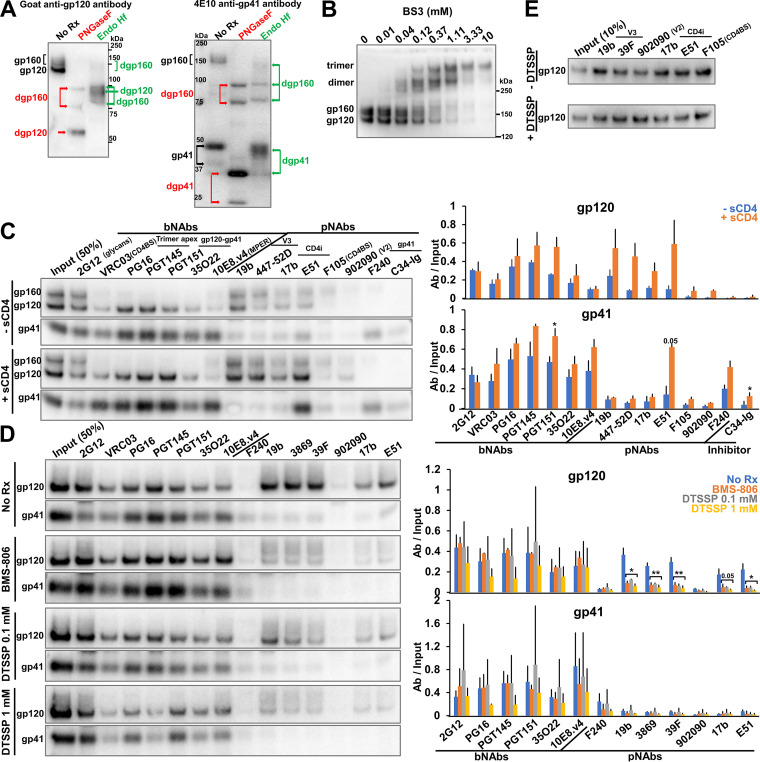
FIG 2.
Characterization of the full-length AD8 Bam Env on IMC-produced virions. (A) 293T cells were transfected with the pNL4-3.AD8 IMC encoding an AD8 Env with Bam (S752F I756F) changes in the cytoplasmic tail (Fig. 3). Forty-eight to seventy-two hours later, the cell supernatants were collected, filtered through a 0.45-μm membrane, and centrifuged at 14,000 or 100,000 × g for 1 h at 4°C (see Materials and Methods). Virus pellets were lysed. Equal volumes of viral lysates were denatured and either untreated or treated with PNGase F or Endo Hf for 1.5 h at 37°C, and Western blotted with a goat anti-gp120 antibody and the 4E10 anti-gp41 antibody. The deglycosylated (dg) Envs produced by PNGase F and Endo Hf are indicated by red and green labels, respectively. (B) Purified virus particles with AD8 Bam Envs were incubated with the BS3 crosslinker at the indicated concentrations for 30 min at room temperature. The samples were subsequently quenched, analyzed by reducing SDS-PAGE and Western blotted with a goat anti-gp120 antibody. (C) Purified virus particles were incubated with a panel of broadly neutralizing antibodies (bNAbs), poorly neutralizing antibodies (pNAbs) and the anti-HR1 C34-Ig peptide for 1 h at room temperature in the presence or absence of 10 μg/mL four-domain soluble CD4 (sCD4). The virus-antibody mixture was diluted 20-fold with 1X PBS and centrifuged. The virus-antibody pellet was lysed and precipitated with protein A-agarose beads for 1 h at 4°C. The beads were washed three times and Western blotted with a goat anti-gp120 antibody and the 4E10 anti-gp41 antibody. (D) Purified virus particles were incubated with 10 μM BMS-806 or with the indicated concentration of DTSSP crosslinker for 30 min at room temperature before the reactions were quenched with 100 mM Tris-HCl, pH 8.0. Env antigenicity on these virus particles was studied as described in panel C. (E) 293T cells were transfected with the pNL4-3.AD8 Bam IMC expressing a soluble version of gp120. Forty-eight hours later, 0.45-μm-filtered supernatant containing the soluble gp120 was crosslinked with 1 mM DTSSP as described above. Aliquots were then incubated with a panel of pNAbs and protein A-agarose beads for 2 h at room temperature before the beads were washed and Western blotted with a goat anti-gp120 antibody. The results shown are representative of those obtained in two independent experiments. The means and standard deviations of the results in C and D are reported in the bar graphs in the panels on the right. The significance of the difference in antibody binding between treated and untreated samples was evaluated by a Student's t test; *, P < 0.05; **, P < 0.01.