Abstract
Aims
Reactive atrial-based anti-tachycardia pacing (rATP) in pacemakers (PMs) and cardiac resynchronization therapy defibrillators (CRT-Ds) has been reported to prevent progression of atrial fibrillation, and this reduced progression is expected to decrease the risk of complications such as stroke and heart failure (HF). This study aimed to assess the cost-effectiveness of rATP in PMs and CRT-Ds in the Japanese public health insurance system.
Methods and results
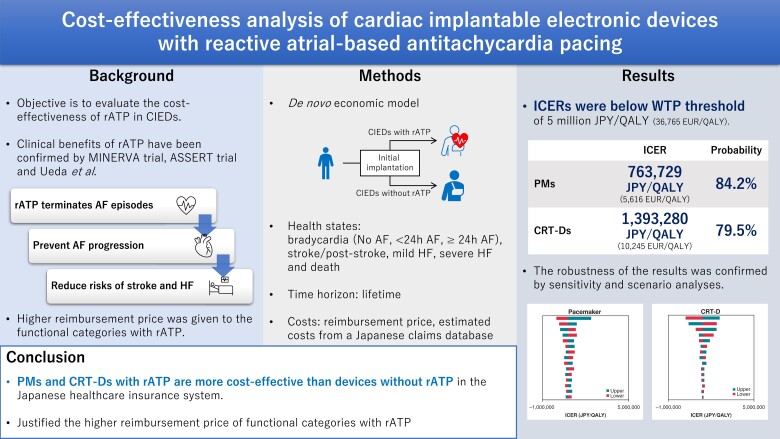
We developed a Markov model comprising five states: bradycardia, post-stroke, mild HF, severe HF, and death. For devices with rATP and control devices without rATP, we compared the incremental cost-effectiveness ratio (ICER) from the payer's perspective. Costs were estimated from healthcare resource utilisation data in a Japanese claims database. We evaluated model uncertainty by analysing two scenarios for each device. The ICER was 763 729 JPY/QALY (5616 EUR/QALY) for PMs and 1,393 280 JPY/QALY (10 245 EUR/QALY) for CRT-Ds. In all scenarios, ICERs were below 5 million JPY/QALY (36 765 EUR/QALY), supporting robustness of the results.
Conclusion
According to a willingness to pay threshold of 5 million JPY/QALY, the devices with rATP were cost-effective compared with control devices without rATP, showing that the higher reimbursement price of the functional categories with rATP is justified from a healthcare economic perspective.
Keywords: Cost-effectiveness, Atrial fibrillation, Heart failure, Pacemaker, Cardiac resynchronization therapy, Atrial anti-tachycardia pacing
Graphical Abstract
Graphical Abstract.
What’s new?
Cost-effectiveness of implantable devices with reactive atrial-based anti-tachycardia pacing (rATP) that can detect atrial fibrillation (AF) and prevent AF progression and subsequent events was evaluated from the Japanese payer’s perspective.
A Markov model was developed to represent pathological changes in patients with a dual-chamber pacemaker (PM) for bradycardia or a cardiac resynchronization therapy defibrillator (CRT-D) for heart failure.
Healthcare resource utilization in patients with a dual-chamber PM or CRT-D device was estimated by using a Japanese claims database.
According to a willingness to pay threshold of 5 million Japanese Yen/quality-adjusted life year, in the Japanese healthcare insurance system dual-chamber PMs and CRT-D devices with rATP are cost-effective compared with control devices without rATP.
Introduction
Atrial fibrillation (AF), a type of tachycardia that causes irregular heart rhythm, is one of the most frequently occurring arrhythmias and most prevalent in people aged 65 years and older; overall, the estimated prevalence in Japan in 2020 was around 0.8–0.9%. The prevalence of AF increases with age, and AF is a major problem in an ageing society.1,2 AF can cause subjective symptoms such as palpitations and chest discomfort, but it can also be asymptomatic. Worsening AF not only reduces quality of life but also increases the risk of stroke and heart failure (HF) deterioration, which leads to a poor prognosis.3 AF has an impact on healthcare system budgets; e.g. in January 2022, AF accounted for almost 1% of total healthcare costs in Japan.4 The prevalence of AF is expected to increase in the future.
AF is a progressive condition that generally increases in duration over time. It is classified according to its duration as paroxysmal, persistent, long-standing persistent or permanent.5 Asymptomatic AF in patients with cardiac implantable electronic devices (CIEDs) and symptomatic AF have been reported to increase the risk of stroke with increasing duration of AF,6 requiring appropriate therapeutic intervention. Recently, quality indicators (QIs) were suggested to evaluate the quality of care for cardiac pacing in CIEDs patients.7 Furthermore, AF is a risk factor for the development of HF, and the two conditions are closely inter-related.8 Patients with AF have been reported to have a worse prognosis when they develop HF.9 Among patients with CIEDs, the complication rate of AF is particularly high in patients with reduced cardiac function and implantation of a cardiac resynchronization therapy defibrillator (CRT-D).10 Deterioration of HF can be fatal, so management of AF is also important in terms of HF management.
The risk of stroke and HF deterioration was reported to increase with increasing duration of AF in patients with CIEDs for bradycardia or HF.6,11 Recently, implantable devices with a reactive atrial-based anti-tachycardia pacing (rATP) algorithm were introduced to prevent AF progressing from paroxysmal to persistent and permanent forms by detecting AF and delivering atrial anti-tachycardia pacing to terminate AF episodes.12 Patients with CIEDs with rATP were reported to have less progression of AF than patients with CIEDs without rATP, and this reduced progression is expected to decrease the risk of complications such as stroke and HF.13–17 Furthermore, in patients with a CRT-D, the risk of hospitalisation for HF is significantly lower in those with rATP device14; a CRT-D with rATP may also prevent deterioration of HF.14 Because stroke and HF have a significant impact on not only patient quality of life but also healthcare costs, devices with rATP may reduce healthcare costs and improve the cost-effectiveness of bradycardia and HF treatment.18
To manage medical technology prices, the Japanese public health insurance system uses a functional category system in which reimbursement prices are set for each functional category instead of each brand; these functional categories are mainly decided on the basis of similarity in structure, purpose of use, and clinical efficacy. Costs for devices in functional categories, e.g. implantable devices, including CIEDs, are paid separately from the cost of the procedure, whereas less expensive or reusable devices in comprehensive categories are paid for inclusively, i.e. they are included in the cost of the procedure. For innovative new medical technologies, new categories are created with premium rates.19 When the clinical effectiveness of rATP was approved by the Japanese regulatory authority in the challenge application programme, novel functional categories with higher reimbursement prices than CIEDs without rATP were established for pacemakers (PMs) and CRT-D devices with rATP.20 However, to the best of our knowledge, no studies have examined the cost-effectiveness of rATP. Therefore, the aim of this study was to assess the cost-effectiveness of rATP in PMs and CRT-D devices and the functional categories of these devices in the Japanese public health insurance system.
Methods
Model structure
In this study, we developed a Markov model to represent pathological changes in patients with a dual-chamber PM for bradycardia or a CRT-D device for HF. The incremental cost-effectiveness ratio (ICER) was calculated by comparing CIEDs with and without rATP from the perspective of the Japanese public health insurance system as the payer. Because reimbursement prices are set for functional categories of devices and not individual devices, target and control devices were defined by using functional categories (Table 1). Reimbursement prices of cardiac resynchronisation therapy pacemakers (CRT-Ps) and implantable cardioverter-defibrillators (ICDs) are same with or without rATP in the Japanese public health insurance system, so it is obviously that CRT-Ps and ICDs with rATP are dominant to those of without rATP in the perspective of cost-effectiveness. Therefore, CRT-Ps and ICDs were not analysed in this study.
Table 1.
Reactive atrial-based anti-tachycardia pacing devices and control devices used in the analysis and their functional categories
| Arm | Functional category | Main definition | Reimbursement price (as of April 2022) |
|---|---|---|---|
| Pacemakers | |||
| rATP | Category 112 pacemaker (3) dual-chamber (Type V) | Has the rATP algorithm | ¥751 000 (€5522) |
| Control | Category 112 pacemaker (2) dual-chamber (Type IV) | Does not have the rATP algorithm | ¥593 000 (€4360) |
| CRT-Ds | |||
| rATP | Category 144 implantable cardioverter-defibrillator with biventricular pacing (2) quadripolar (c) with rATP algorithm | CRT-D meets the following definitions: − Has the algorithm that dynamically adjusts CRT pacing parameters (optimisation algorithm with automated pacing parameters and RV-synchronized, LV-only pacing): − Has the rATP algorithm |
¥4 750 000 (€34 926) |
| Control | Category 144 implantable cardioverter-defibrillator with biventricular pacing (2) quadripolar (b) with automated optimisation algorithm | CRT-D meets the following definitions: − Has the algorithm that dynamically adjusts CRT pacing parameters (optimization algorithm with automated pacing parameters and RV-synchronized, LV-only pacing): − Does not have the rATP algorithm |
¥4 410 000 (€32 426) |
CRT-D, cardiac resynchronization therapy defibrillator; LV, left ventricle; rATP, reactive atrial-based anti-tachycardia pacing; RV, right ventricle; €1 = \136 (monthly exchange rate announced by Bank of Japan on 20 September 2022).
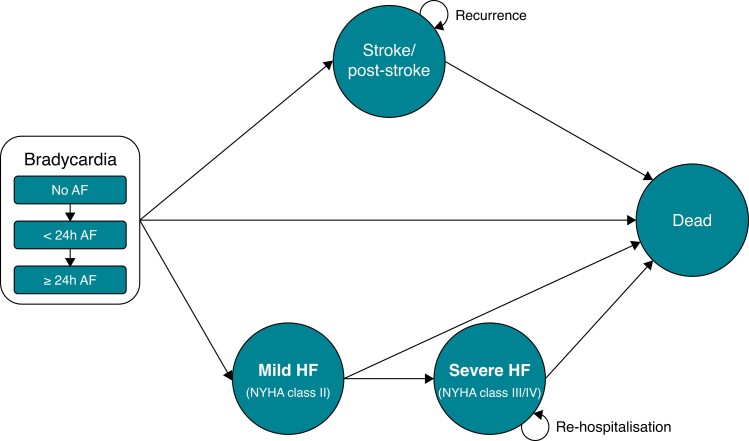
The Markov model comprised five states: bradycardia, stroke/post-stroke, mild HF [New York Heart Association (NYHA) Class II], severe HF (NYHA Class III/IV), and death. Onset of stroke and HF were assumed to be irreversible and to not occur simultaneously, so patients in the stroke/post-stroke and severe HF states could progress to death only and those in the mild HF could progress to severe HF or death. On the basis of the results of Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT),13 a randomized controlled study on the relationship between AF progression and risk of stroke and HF, and the Minimize Right Ventricular Pacing to Prevent Atrial Fibrillation and Heart Failure (MINERVA) trial, the first randomized controlled trial to show the effectiveness of rATP in preventing progression of AF, we defined three substates of bradycardia: no AF, AF lasting <24 h and AF lasting 24 h or longer (Figure 1).15
Figure 1.
Markov model diagram. For the pacemaker analysis, all patients were entered into the model in the bradycardia without atrial fibrillation, and no replacement with cardiac resynchronization therapy was performed in case of transition to heart failure; for cardiac resynchronisation therapy device, all patients were entered into the model in the mild HF state. AF, atrial fibrillation; HF, heart failure.
We did not include adverse events in the analysis because we assumed that incidences of adverse events would be similar with rATP and control devices.14,15
We used a common model for the PM and CRT-D analyses. For the PM analysis, all patients were entered into the model in the state of bradycardia with no AF and assumed that the PM was not replaced with a CRT-D in case of transition to HF; for the CRT-D analysis, we entered all patients into the model in the state of mild HF (NYHA Class II).
The time horizon was set to the period until patients reached the age of 100 years and 99.9% of patients were dead (equivalent to a lifetime horizon). The model cycle was set at 1 month, and a half-cycle correction was performed. Costs and utilities were discounted at a rate of 2% per annum according to the Japanese cost-effectiveness analysis guideline of the Center for Outcomes Research and Economic Evaluation for Health (C2H), National Institute of Public Health.21
The present study utilized the data obtained from the ASSERT trial,6,11,13 MINERVA trial,15 RAFT (Resynchronization for Ambulatory Heart Failure Trial),22 Ueda et al.14 and Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure (COMPANION) trial.23,24 The ASSERT trial, MINERVA trial, RAFT, and COMPANION trial had been registered at ClinicalTrials.gov as NCT00256152, NCT00262119, NCT00251251, and NCT00180258, respectively. All trials were approved by the ethics committees of all participating centres and were performed in compliance with the Declaration of Helsinki. The study protocol of Ueda et al. was approved by the institutional review board of the National Cerebral and Cardiovascular Center, Japan (M26-150-6). Check list of the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) is presented in Supplementary material online, Table S1.25
Model inputs
The parameters used in the model are summarized in Table 2. For almost all transition probabilities, we used results of clinical trials conducted outside Japan, but we based cost and utility values on the results of clinical trials or database analyses performed in Japan.
Table 2.
Values and distributions of model parameters
| Parameter | Base case value | Distribution | Source | |
|---|---|---|---|---|
| rATP arm | Control arm | |||
| (a) Pacemakers | ||||
| Background | ||||
| ȃAge (years) | 79.3 | — | Log-normal | NDB Open Data26 |
| ȃMale (%) | 47.0% | — | None | NDB Open Data26 |
| Initial distribution | — | |||
| ȃNo AF | 100.00% | — | None | Assumption |
| ȃ<24 h AF | 0.00% | — | None | Assumption |
| ȃ≥24 h AF | 0.00% | — | None | Assumption |
| ȃMild HF | 0.00% | — | None | Assumption |
| ȃSevere HF | 0.00% | — | None | Assumption |
| Transition rate (/month) | ||||
| ȃNo AF to no AF | 97.59% | — | Beta | Assumption |
| ȃNo AF to <24 h AF | 1.58% | — | Beta | Wong et al. 13 |
| ȃNo AF to ≥24 h AF | 0.46% | — | Beta | Wong et al.13 |
| ȃNo AF to stroke | 0.05% | — | Beta | Van Gelder et al. 6 |
| ȃNo AF to mild HF | 0.19% | — | Beta | Healey et al.11 |
| ȃNo AF to death | 0.13% | — | Beta | Gonzalez et al.27 |
| ȃ<24 h AF to <24 h AF | 98.68% | 98.20% | Beta | Assumption |
| ȃ<24 h AF to ≥24 h AF | 0.85% | 1.29% | Beta | Wong et al.13 Borian et al.15 |
| ȃ<24 h AF to stroke | 0.08% | — | Beta | Van Gelder et al. 6 |
| ȃ<24 h AF to mild HF | 0.21% | — | Beta | Wong et al.13 |
| ȃ<24 h AF to death | 0.18% | 0.22% | Beta | Borian et al.15 |
| ȃ≥24 h AF to ≥24 h AF | 98.81% | 98.77% | Beta | Assumption |
| ȃ≥24 h AF to stroke | 0.24% | — | Beta | Van Gelder et al.6 |
| ȃ≥24 h AF to mild HF | 0.77% | — | Beta | Wong et al. 201813 |
| ȃ≥24 h AF to death | 0.18% | 0.22% | Beta | Borian et al.15 |
| ȃStroke to stroke | 99.82% | 99.78% | Beta | Assumption |
| ȃStroke to death | 0.18% | 0.22% | Beta | Borian et al.15 |
| ȃMild HF to mild HF | 99.15% | 98.80% | Beta | Assumption |
| ȃMild HF to severe HF | 0.26% | 0.61% | Beta | Tang et al.22 Ueda et al.14 |
| ȃMild HF to death | 0.59% | — | Beta | Tang et al.22 |
| ȃSevere HF to severe HF | 98.07% | — | Beta | Assumption |
| ȃSevere HF to death | 1.93% | — | Beta | Carson et al. 23 |
| Re-hospitalization rate (/month) | ||||
| ȃMild HF | 0.00% | — | None | Assumption |
| ȃSevere HF | 0.62% | 1.45% | Beta | Tang et al.22 Ueda et al.14 |
| Recurrent rate (/month) | ||||
| ȃStroke | 0.27% | — | Beta | Takashima et al.28 |
| Acute death (/month) | ||||
| ȃHF | 8.70% | — | Beta | Sasaki et al.29 |
| ȃStroke | 4.92% | — | Beta | Toyoda et al.27 |
| Acute phase cost (/month) | ||||
| ȃInitial implantationa | ¥2 265 928 (€16 661) |
¥2 107 928 (€15 499) |
Gamma | MDV database30 |
| ȃStroke | ¥2 038 484 (€14 989) |
— | Gamma | MDV database30 |
| ȃHF | ¥1 016 617 (€7475) |
— | Gamma | MDV database30 |
| ȃDevice replacementa | ¥1 685 386 (€12 393) |
¥1 527 386 (€11 231) |
Gamma | MDV database30 |
| Follow-up cost (/month) | ||||
| ȃMild/severe HF | ¥47 188 (€347) |
— | Gamma | MDV database30 |
| ȃStroke | ¥18 998 (€140) |
— | Gamma | MDV database30 |
| Utility | — | |||
| ȃNoAF/<24 h AF/≥24 h AF | 0.897 | — | Beta | Shiroiwa et al.31 |
| ȃStroke | 0.632 | — | Beta | Shiroiwa et al.31 |
| ȃMild HF | 0.878 | — | Beta | Göhler et al.32 |
| ȃSevere HF | 0.768 | — | Beta | Göhler et al.32 |
| Device replacement (year/time) | ||||
| ȃPM | 13.70 | — | Gamma | Product catalogue33 |
| Effect ratio—rATP | ||||
| ȃAF extension | 0.659 | NA | Beta | Borian et al.15 |
| ȃAF mortality | 0.818 | NA | Beta | Gonzalez et al.27 |
| ȃStroke mortality | 0.818 | NA | Beta | Gonzalez et al.27 |
| ȃHF deterioration | 0.426 | NA | Beta | Ueda et al.14 |
| ȃDiscount rate | 2.00% | — | None | C2H guideline21 |
| (b) Cardiac resynchronization therapy defibrillators | ||||
| Background | ||||
| ȃAge (years) | 69.1 | — | Log-normal | NDB Open Data26 |
| ȃMale (%) | 75.6% | — | None | NDB Open Data26 |
| Initial distribution | ||||
| ȃMild HF | 100.00% | — | None | Assumption |
| ȃSevere HF | 0.00% | — | None | Assumption |
| Transition rate (/month) | ||||
| ȃMild HF to mild HF | 99.39% | 99.14% | Beta | Assumption |
| ȃMild HF to severe HF | 0.19% | 0.44% | Beta | Tang et al.22 Ueda et al.14 |
| ȃMild HF to death | 0.42% | — | Beta | Tang et al.22 |
| ȃSevere HF to severe HF | 98.79% | — | Beta | Assumption |
| ȃSevere HF to death | 1.21% | — | Beta | Carson et al.23 |
| Re-hospitalisation rate (/month) | ||||
| ȃMild HF | 0.00% | — | None | Assumption |
| ȃSevere HF | 0.41% | 0.95% | Beta | Tang et al.22 Ueda et al.14 |
| Acute death (/month) | ||||
| ȃHF | 8.70% | — | Beta | Sasaki et al.29 |
| ȃAcute phase cost (/month) | — | |||
| ȃInitial implantationa | ¥7 610 493 (€55 960) |
¥7 950 493 (€58 460) |
Gamma | MDV database30 |
| ȃHF | ¥1 090 732 (€8020) |
— | Gamma | MDV database30 |
| ȃDevice replacementa | ¥5 764 311 (€42 385) |
¥6 104 311 (€44 885) |
Gamma | MDV database30 |
| Follow-up cost (/month) | ||||
| ȃMild/severe HF | ¥43 154 (€317) |
— | Gamma | MDV database30 |
| Utility | ||||
| ȃMild HF | 0.884 | — | Beta | Göhler et al.32 |
| ȃSevere HF | 0.768 | — | Beta | Göhler et al.32 |
| Device replacement (year/time) | ||||
| ȃCRT-D | 9.20 | — | Gamma | Product catalogue34 |
| Effect ratio—rATP | ||||
| ȃHF deterioration | 0.432 | NA | Beta | Ueda et al.14 |
| ȃDiscount rate | 2.00% | — | None | C2H guideline21 |
AF, atrial fibrillation; HF, heart failure; rATP; reactive atrial-based anti-tachycardia pacing; —, same value as rATP arm.
Device reimbursement price is included; €1 = 136 (monthly exchange rate announced by Bank of Japan on 20 September 2022).
Modelled population
The baseline patient characteristics were the same as those of users of dual-chamber PMs and CRT-Ds in the National Database (NDB) Open Data in 2019, a Japanese nationwide claims database.26 The mean age of PM users was 79.3 years, and 47.0% of them were male and the mean age of CRT-D users was 69.1 years and 75.6% were male.
Transition probabilities
In patients with bradycardia, transition probabilities for AF progression (i.e. transitions to a more severe AF substate) were based on the results of ASSERT13; for the rATP device, the relative reduction in risk of AF progression was set to the value reported in the MINERVA trial.15 The risk of HF and stroke by AF bradycardia substate was also obtained from ASSERT.6,11,13 Any patients with bradycardia who progressed to HF were assumed to transition to the mild HF state. Because HF hospitalization indicates worsening HF, the probability of transition from mild HF to severe HF was set to the incidence of HF hospitalization reported in RAFT,22 a randomized trial conducted in the US that compared implantable cardioverter-defibrillators with and without CRT. The relative reduction in risk of HF hospitalization with rATP was based on the result of a single-centre, retrospective study performed by Ueda et al.14
Regarding mortality in patients with bradycardia, we used the results of Gonzalez et al.27 for patients without AF and those of the MINERVA trial15 for patients with AF. Mortality in mild HF was based on the RAFT22 results and that in severe HF on the results of the COMPANION trial,23,24 a randomized controlled study conducted in the US that reported mortality in patients with advanced HF who were using a CRT-D. In addition, we assumed that a certain proportion of patients died in the acute phase of stroke or HF. Acute mortality of HF was obtained from Sasaki et al.29 and that of stroke from Toyoda et al.35 Patients in a stroke/post-stroke state were assumed to experience recurrence, and the rates were obtained from Takashima et al.28 Patients with severe HF were assumed to experience HF re-hospitalization, and the rate was obtained from RAFT22 and converted to a rate per patient-month. Acute mortality was also assumed in patients who experienced recurrence of stroke or re-hospitalization for HF. Natural mortalities were based on the 2020 Japanese life tables,36 adjusted for sex and age. The initial value of the sex ratio was assumed to remain constant over the entire period.
Costs and utility values
The price of devices with rATP and control devices were the reimbursement prices of the corresponding functional categories as of April 2022. We estimated costs other than the device prices on the basis of healthcare resource utilisation in real clinical settings. Healthcare resource utilisation was estimated by using Medical Data Vision (MDV) database,30 a Japanese claims database (see Supplementary material online, Table S2 for details). Data on all patients in whom CIEDs were implanted from April 2018 to December 2020 were extracted and the following costs were estimated: (i) cost at the time of device implantation, (ii) cost at time of device replacement, (iii) cost of hospitalization for acute HF, (iv) cost of hospitalization for acute stroke, and (v) monthly cost of follow-up for HF and stroke. Hospitalization for acute HF was defined as inpatient claims for brain natriuretic peptide or N-terminal pro-brain natriuretic peptide testing after initial hospitalization and hospitalization for acute stroke as use of tissue plasminogen activator or stoke-related medical devices or both.
Cost of acute hospitalization and monthly costs of follow-up were assumed to be common to rATP and control devices. For rATP devices, costs at the time of device implantation and device replacement were calculated by adding the difference in reimbursement price between rATP devices and control devices to the costs for control devices because there should be no difference in costs for implantation procedures, treatments for controlling adverse events or device replacement between devices with and without rATP. For battery longevity, we used the product specification values for the PMs and CRTs with rATP.33,34 We assumed the same battery longevity for devices with and without rATP because rATP has only a minor impact on battery longevity.14
The utility values for patients with bradycardia were assumed to be equivalent to those of healthy individuals corrected for age at baseline, as estimated by Shiroiwa et al.31 In stroke patients, the utility values were calculated by subtracting their disutility values, which were also estimated by Shiroiwa et al.,31 from the utility values of healthy individuals. The utility values for patients with HF were obtained from Göhler et al.32 who examined the utility values according to NYHA class with a regression model that considered patient background. In the present model the utility values for each NYHA class were calculated by using coefficients reported in Göhler et al.,32 i.e. the age and sex ratio at baseline in the present study, as described above. For the ratio of NYHA III to NYHA IV in patients with severe HF, we used the values from the COMPANION trial.23,24 We did not consider disutility according to the progression of AF because of a lack of published studies.
Sensitivity and scenario analyses
We performed deterministic sensitivity analyses (DSAs) and probabilistic sensitivity analyses (PSAs) to assess the uncertainty of base case results and selected parameters to cover the uncertainty. To avoid devices with rATP being less effective than control devices, we varied the ratio of transition probabilities rather than the transition probabilities themselves. Similarly, for the cost of rATP devices, we varied the difference with respect to control devices. In DSA, sufficiently large variation was considered to be plus or minus 5 years for age and 20% for other parameters, and the discount rate was varied from 0% to 4% in accordance with the C2H guideline.21 PSA were performed with a Monte Carlo simulation with 3000 samples. The distributions were chosen by the nature of the parameters and are shown in Table 2. The results of the DSA were plotted as Tornado diagrams and that of the PSA as a cost-effectiveness plane and acceptability curve.
To evaluate the uncertainty introduced by the assumption that all patients entered the model in the bradycardia substate without AF for the PM analysis and in the mild HF state for the CRT-D analysis, we performed an analysis of two scenarios for each category in which we used a modified initial distribution of AF substates for the PMs and an initial distribution of NYHA class for the CRT-Ds (Table 3).
Table 3.
Values for scenario analysis
| No AF | <24 h AF | ≥24 h AF | Source | |
|---|---|---|---|---|
| (a) Pacemakers | ||||
| Base case | 100.0% | 0.0% | 0.0% | Assumption |
| Scenario 1 | 79.1% | 9.3% | 11.6% | Connolly et al.37 Botto et al.38 |
| Scenario 2 | 53.0% | 20.9% | 26.1% | Botto et al.38 Lamas et al.39 |
Results
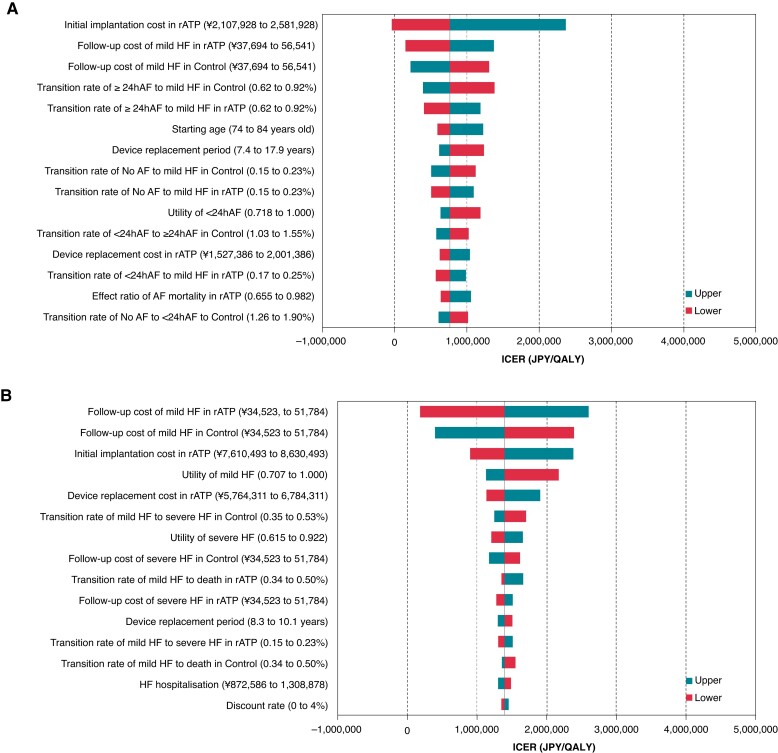
The ICER for PMs with rATP was 763 729 Japanese Yen (JPY)/QALY (5616 EUR/QALY) and for CRT-Ds with rATP was 1 393 280 JPY/QALY (10 245EUR/QALY). Both values were below the willingness to pay threshold of 5 million JPY/QALY (36 765 EUR/QALY) (Table 4).41 The results of the DSA showed that for PMs the variables with a significant impact on ICER were the device costs, chronic monthly healthcare costs for mild HF and the risk of developing HF from an AF state lasting more than 24 h; for CRT-Ds the variables were the device costs and chronic monthly healthcare costs for mild HF, device costs and utility values for patients with mild HF. However, the maximum ICER for all of these variables was below 5 million JPY/QALY (Figure 2).
Table 4.
Base case results
| Total costs | Total QALYs | ΔCosts | ΔQALYs | ICER (/QALY) | |
|---|---|---|---|---|---|
| (a) Pacemaker | |||||
| rATP | ¥3 826 939 (€28 139) | 7.22 | 50 028 (€1103) | 0.20 | 763 729 (€5616) |
| Control | ¥3 676 911 (€27 036) | 7.03 | |||
| (b) Cardiac resynchronization therapy device | |||||
| rATP | ¥15 923 150 (€117 082) | 7.69 | 956 973 (€7037) | 0.69 | 1 393 280 (€10 245) |
| Control | ¥14 966 178 (€110 045) | 7.01 | |||
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; rATP; reactive atrial-based anti-tachycardia pacing; €1 = 136.
Figure 2.
Tornado diagram. (A) Pacemaker. AF, atrial fibrillation; HF, heart failure; ICER, incremental cost-effectiveness ratio; JPY, Japanese yen; QALY, quality-adjusted life year; rATP, reactive atrial-based anti-tachycardia pacing. (B) Cardiac resynchronisation therapy device. AF, atrial fibrillation; HF, heart failure; ICER, incremental cost-effectiveness ratio; JPY, Japanese yen; QALY, quality-adjusted life year; rATP, reactive atrial-based anti-tachycardia pacing.
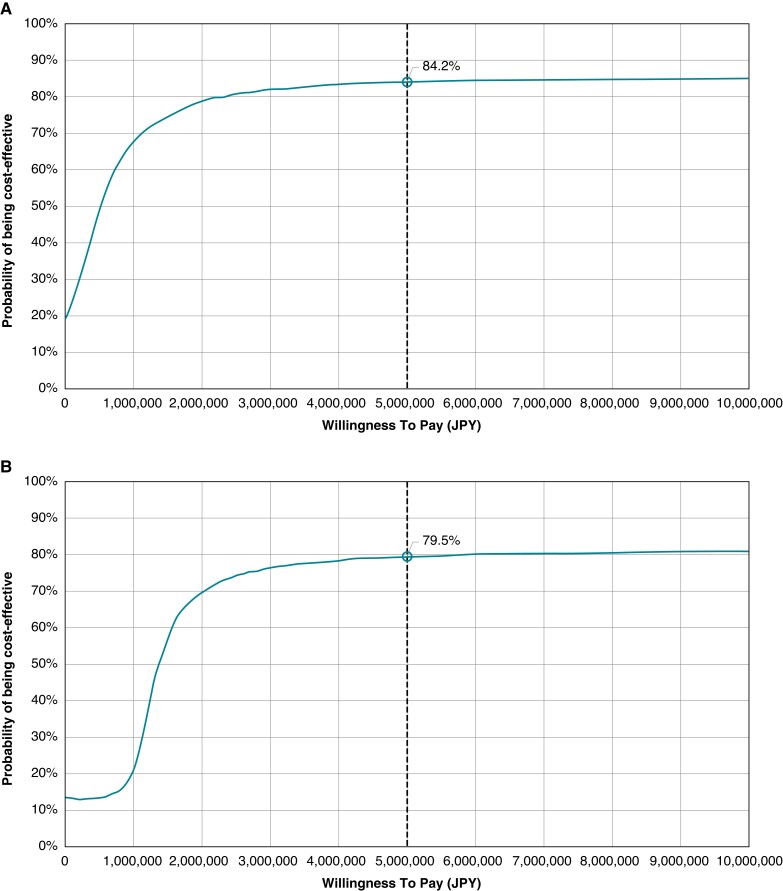
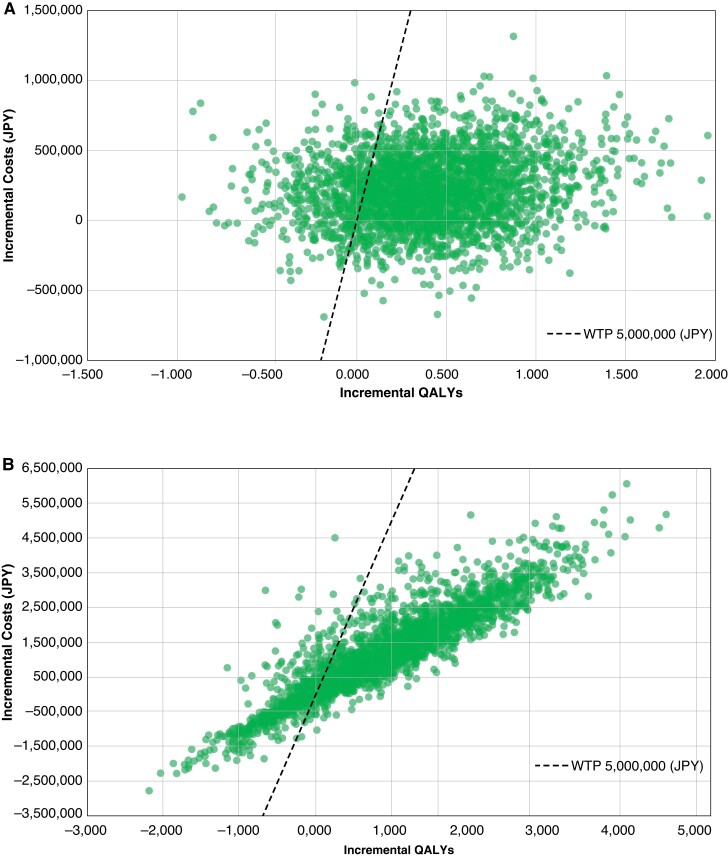
The results from the PSA are shown in Figures 3 and 4. The analyses found that the probability of ICER being below the willingness to pay threshold was 84.2% for PMs and 79.5% for CRT-Ds.
Figure 3.
Cost-effectiveness acceptability curve. (A) Pacemaker. JPY, Japanese yen. (B) Cardiac resynchronization therapy device. JPY, Japanese yen.
Figure 4.
Incremental cost-effectiveness ratio scatter. (A) Pacemaker. ICER, incremental cost-effectiveness ratio; JPY, Japanese yen; QALYs, quality-adjusted life years; WTP, willingness to pay. (B) Cardiac resynchronisation therapy device. ICER, incremental cost-effectiveness ratio; JPY, Japanese yen; QALYs, quality-adjusted life years; WTP, willingness to pay.
For PMs the ICERs in the scenario analysis were 712 412 JPY/QALY for scenario 1 and 660 698 JPY/QALY for scenario 2 and for CRT-Ds they were 1 454 653 JPY/QALY for Scenario 1 and 1 521 943 JPY/QALY for Scenario 2. The ICERs were below 5 million JPY/QALY in all scenarios.
Discussion
To our knowledge, this study was the first to assess the cost-effectiveness of PMs and CRT-Ds with rATP.
In this study, any other treatment for AF performed as part of standard clinical practice, such as catheter ablation, antiarrhythmic drug administration or electrical cardioversion, was not included. Rate control and rhythm control are both used for the treatment of AF, but recent studies have reported some clinical advantages of the latter.42,43 Catheter ablation is the leading rhythm control treatment for AF and its effectiveness is well established.44,45 However, in some cases catheter ablation cannot completely control AF,46 and its complications are of concern in older patients47 and patients who subsequently develop atrial tachycardia.48 Therefore, rATP is expected to be effective as an additional or alternative treatment to catheter ablation. Indeed, the efficacy of rATP has been reported in preventing persistent AF in PM15,49 and HF in CRT14 so improvement of the treatment and prognosis in patients with CIED is expected. The effectiveness of rATP has been demonstrated irrespective of AF duration and device model,49 making it highly versatile. Also, rATP is safer than catheter ablation or drug therapy because there are no complications or concerns about adverse effects.14,15 Therefore, the use of rATP as an alternative treatment in patients in whom catheter ablation should be carefully considered, such as older adults, is also expected to reduce the cost of catheter ablation treatment. Recently, AF was also reported to be associated with a risk of cognitive impairment and dementia,50,51 and catheter ablation was reported to potentially reduce those risks52; suppression of AF progression with rATP is also expected to reduce these risks.
Although the cost-effectiveness of PM is well studied,53–55 the effect of AF prolongation has rarely been considered. In their cost-effectiveness analysis of PM, Rinfret et al.53 adopted the non-fatal cardiac incident rate, a composite parameter including AF, new HF and stroke. Edwards et al.54 performed a cost-effectiveness analysis with a Markov model in patients with symptomatic bradycardia to compare single-chamber PM with dual-chamber PM. Like ours, their model incorporated AF (paroxysmal and chronic AF), stroke/post-stroke and HF as states in the model; the additional benefit of dual-chamber PM was implemented in the model by using the result of the DANPACE trial,54 which showed that the odds ratio for paroxysmal AF is lower in patients with a dual-chamber PM than in those with a single-chamber PM. However, the incidence of HF, stroke and chronic AF were not significantly different between single-chamber PM and dual-chamber PM.54 The results of the DSA by Edwards et al. showed that the variable that affected ICER most was the price of PM. The cost-effectiveness of CRT has also been extensively studied,56,57 and some of the studies developed models to classify HF by severity. However, none of them examined the deterioration of HF due to the AF progression.
This is the first study to model the effect of rATP on prevention of AF progress and show that the ICERs for PM and CRT-D with rATP were below the Japanese willingness to pay threshold of 5 million JPY/QALY when using the reimbursement prices as of April 2022. Sensitivity and scenario analyses confirmed the robustness of the results. In DSA (Figure 2), the variable that most affected the ICER was the price of the device, which is consistent with Edwards et al.54 The impact of the uncertainty of variables that represent an additional benefit of rATP (i.e. the risk of AF progression, HF deterioration, and HF re-hospitalization) was relatively small. We varied the additional cost of devices with rATP with respect to control devices from 0 JPY to 474 000 JPY in PM and from 0 JPY to 1 020 000 JPY in CRT-D, which is large enough in view of the variation expected in the Japanese public healthcare system, and found that the ICER of devices with rATP was lower than 5 million JPY/QALY.
Limitations
First, although we evaluated the cost-effectiveness of rATP devices implanted in Japanese patients, for some parameters we used the results of trials performed outside Japan because of a lack of data from Japanese studies. For example, we used the mortality in patients with HF from RAFT and the COMPANION trial, which were conducted in US patients, who have different backgrounds from Japanese patients on CRT: Japanese patients receiving CRT have a higher left ventricle ejection fraction, so preserved cardiac function is more common among patients in Japan than among those in the US. In addition, the proportion of patients with ischaemic heart disease is lower in Japan than in the populations studied in the above-mentioned trials.22–24 Furthermore, the present study may have overestimated mortality rates because it analysed data from 2020 by using mortality rates from RAFT and the COMPANION trial, which were published in 2010 and 2004, respectively, and the performance of CRT and drugs has improved since the publication of those trials. However, according to the results of sensitivity analysis, these is no significant impact of transition probabilities from non-Japanese clinical trials on ICERs, the extrapolation of non-Japanese clinical trials would not have a significant impact on the conclusion.
Second, we assumed that patient mortality after stroke is the same as that of patients with bradycardia even though it might be higher. We also assumed that there is no disutility for AF progression. We performed DSA and PSA to assess the impact of uncertainty on the results and found that the uncertainty had a negligible impact on the conclusions.
Third, we did not consider whether any treatment for AF performed as part of standard clinical practice, such as catheter ablation, antiarrhythmic drug administration or electrical cardioversion, may be different between patients with devices with and without rATP.
Fourth, in real clinical practice, treatment for AF is not limited to rATP so rATP may actually have a smaller effect on preventing AF progression. However, the results of the DSA indicated that the uncertainty in the ratio of transition probability for devices with rATP to those for control devices was small.
Fifth, although tachycardiomyopathy (TCT) is partially or completely reversible after treatment of the triggering arrhythmia,58,59 as far as we know, no clinical evidence has shown the relation between prevention of AF progression by rATP and TCT. Therefore, we considered that it is difficult to include the reversibility in this study at this time.
Last, although battery longevity can vary depending on individual patient conditions, we used the standard value from the product specifications.
Conclusion
PMs and CRT-Ds with rATP are more cost-effective than devices without rATP in the Japanese healthcare insurance system, which justifies the higher reimbursement price of these functional categories with rATP from the perspective of healthcare economics.
Supplementary Material
Contributor Information
Takashi Noda, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, 6-1 Kishibe-Shimmachi, Suita, Osaka 564-8565, Japan; Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine and Tohoku University Hospital, 1-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8574, Japan.
Nobuhiko Ueda, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, 6-1 Kishibe-Shimmachi, Suita, Osaka 564-8565, Japan.
Yuji Tanaka, Healthcare Economics and Government Affairs, Medtronic Japan Co., Ltd., 1-2-70 Konan, Minato-ku, Tokyo 108-0075, Japan.
Yoko Ishiguro, Healthcare Economics and Government Affairs, Medtronic Japan Co., Ltd., 1-2-70 Konan, Minato-ku, Tokyo 108-0075, Japan.
Tomoko Matsumoto, Healthcare Economics and Government Affairs, Medtronic Japan Co., Ltd., 1-2-70 Konan, Minato-ku, Tokyo 108-0075, Japan.
Tatsuhiro Uenishi, Data Science Department, Medilead, Inc., 3-20-2 Nishi-Shinjuku, Shinjuku-ku, Tokyo 163-1424, Japan.
Hiroko Yamaguchi, Data Science Department, Medilead, Inc., 3-20-2 Nishi-Shinjuku, Shinjuku-ku, Tokyo 163-1424, Japan.
Ayako Shoji, Data Science Department, Medilead, Inc., 3-20-2 Nishi-Shinjuku, Shinjuku-ku, Tokyo 163-1424, Japan; Healthcare Consulting Inc., 1-8-19 Fujimi, Chiyoda-ku, Tokyo 102-0071, Japan.
Jae-Eun Myung, Government Affairs and Market Access, Medtronic Korea Ltd., #534, Teheran-ro, Gangnam-gu, Seoul 06181, Korea; Department of Pharmaceutical Medicine and Regulatory Science, College of Medicine and Pharmacy, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea.
Kengo Kusano, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, 6-1 Kishibe-Shimmachi, Suita, Osaka 564-8565, Japan.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was funded by Medtronic Japan.
Data availability
The claims database that supports the findings of this study are available from Medical Data Vision Co., Ltd. but were used under licence for the current study; therefore, restrictions apply, and the data are not publicly available. For inquiries about access to the data set used in this study, please contact MDV (https://www.mdv.co.jp/; email address, ebm_sales@mdv.co.jp).
Other data underlying this article are available in the article and in its online Supplementary material.
References
- 1. Ohsawa M, Okayama A, Sakata K, Kato K, Itai K, Onoda Tet al. Rapid increase in estimated number of persons with atrial fibrillation in Japan: an analysis from national surveys on cardiovascular diseases in 1980, 1990 and 2000. J Epidemiol 2005;15:194–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota Iet al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol 2009;137:102–7. [DOI] [PubMed] [Google Scholar]
- 3. Martinez C, Katholing A, Freedman SB. Adverse prognosis of incidentally detected ambulatory atrial fibrillation. A cohort study. Thromb Haemost 2014;112:276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Federation of Health Insurance Societies (KENPOREN) . Trend survey on the top 30 diseases in medical expenses in January 2022. (in Japanese). https://www.kenporen.com/toukei_data/pdf/chosa_r04_06_05.pdf
- 5. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DLet al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation [published correction appears in Eur Heart J. 2021 May 14; 42(19):1908] [published correction appears in Eur Heart J. 2021 May 14; 42(19):1925] [published correction appears in Eur Heart J. 2021 May 13]. Eur Heart J 2021;42:1289–367.32860058 [Google Scholar]
- 6. Van Gelder IC, Healey JS, Crijns HJGM, Wang J, Hohnloser SH, Gold MRet al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–44. [DOI] [PubMed] [Google Scholar]
- 7. Aktaa S, Abdin A, Arbelo E, Burri H, Vernooy K, Blomström-Lundqvist Cet al. European Society of Cardiology Quality Indicators for the care and outcomes of cardiac pacing: developed by the Working Group for Cardiac Pacing Quality Indicators in collaboration with the European Heart Rhythm Association of the European Society of Cardiology. Europace 2022;24:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? . Eur Heart J 2015;36:3250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PAet al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–5. [DOI] [PubMed] [Google Scholar]
- 10. Baker DW, Wright RF. Management of heart failure. IV. Anticoagulation for patients with heart failure due to left ventricular systolic dysfunction. JAMA 1994;272:1614–8. [DOI] [PubMed] [Google Scholar]
- 11. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci Aet al. Subclinical atrial fibrillation and the risk of stroke [published correction appears in N Engl J Med. 2016 Mar 10; 374(10):998]. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 12. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy [published correction appears in Europace. 2022 Mar 07]. Europace 2022;24:71–164. [DOI] [PubMed] [Google Scholar]
- 13. Wong JA, Conen D, Van Gelder IC, McIntyre WF, Crijns HJ, Wang Jet al. Progression of device-detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol 2018;71:2603–11. [DOI] [PubMed] [Google Scholar]
- 14. Ueda N, Kamakura T, Noda T, Nakajima K, Kataoka N, Wada Met al. Efficacy and safety of new-generation atrial antitachycardia pacing for atrial tachyarrhythmias in patients implanted with cardiac resynchronization therapy devices. J Cardiol 2020;75:559–66. [DOI] [PubMed] [Google Scholar]
- 15. Boriani G, Tukkie R, Manolis AS, Mont L, Pürerfellner H, Santini Met al. Atrial antitachycardia pacing and managed ventricular pacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: the MINERVA randomized multicentre international trial. Eur Heart J 2014;35:2352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boriani G, Pieragnoli P, Botto GL, Puererfellner H, Mont L, Ziacchi Met al. Effect of PR interval and pacing mode on persistent atrial fibrillation incidence in dual chamber pacemaker patients: a sub-study of the international randomized MINERVA trial. Europace 2019;21:636–44. [DOI] [PubMed] [Google Scholar]
- 17. Padeletti L, Pürerfellner H, Mont L, Tukkie R, Manolis AS, Ricci Ret al. New-generation atrial antitachycardia pacing (Reactive ATP) is associated with reduced risk of persistent or permanent atrial fibrillation in patients with bradycardia: results from the MINERVA randomized multicenter international trial. Heart Rhythm 2015;12:1717–25. [DOI] [PubMed] [Google Scholar]
- 18. Boriani G, Manolis AS, Tukkie R, Mont L, Pürerfellner H, Santini Met al. Effects of enhanced pacing modalities on health care resource utilization and costs in bradycardia patients: an analysis of the randomized MINERVA trial. Heart Rhythm 2015;12:1192–200. [DOI] [PubMed] [Google Scholar]
- 19. Tamura M, Nakano S, Sugahara T. Reimbursement pricing for new medical devices in Japan: is the evaluation of innovation appropriate? . Int J Health Plann Manage 2019;34:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Myung JE, Tanaka Y, Choi H, Strachan L, Watanuki T, Lee JHet al. Coverage with evidence development programs for medical technologies in Asia-pacific regions: A case study of Japan and South Korea. JMA J 2021;4:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Center for Outcomes Research and Economic Evaluation for Health, National Institute of Public Health (C2H) . Guideline for Preparing Cost-Effectiveness Evaluation to the Central Social Insurance Medical Council. Version 3.0 19th January, 2022. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf
- 22. Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly Set al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010;363:2385–95. [DOI] [PubMed] [Google Scholar]
- 23. Carson P, Anand I, O'Connor C, Jaski B, Steinberg J, Lwin Aet al. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial [published correction appears in J Am Coll Cardiol.2008 Jun 3; 51(22): 2197. Ghali, Jalil [corrected to Ghali, Jalal]]. J Am Coll Cardiol 2005;46:2329–34. [DOI] [PubMed] [Google Scholar]
- 24. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco Tet al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–50. [DOI] [PubMed] [Google Scholar]
- 25. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell Cet al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health 2022;25:3–9. [DOI] [PubMed] [Google Scholar]
- 26. Ministry of Health, Labour and Welfare . NDB open data in 2019. (in Japanese). https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000177221_00010.html.
- 27. Gonzalez M, Keating RJ, Markowitz SM, Liu CF, Thomas G, Ip JEet al. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm 2014;11:2214–21. [DOI] [PubMed] [Google Scholar]
- 28. Takashima N, Arima H, Kita Y, Fujii T, Tanaka-Mizuno S, Shitara Set al. Two-year recurrence after first-ever stroke in a general population of 1.4 million Japanese patients- the shiga stroke and heart attack registry study. Circ J 2020;84:943–8. [DOI] [PubMed] [Google Scholar]
- 29. Sasaki N, Kunisawa S, Ikai H, Imanaka Y. Differences between determinants of in-hospital mortality and hospitalisation costs for patients with acute heart failure: a nationwide observational study from Japan. BMJ Open 2017;7:e013753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laurent T, Simeone J, Kuwatsuru R, Hirano T, Graham S, Wakabayashi Ret al. Context and considerations for use of two Japanese real-world databases in Japan: medical data vision and Japanese medical data center. Drugs Real World Outcomes 2022;9:175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shiroiwa T, Noto S, Fukuda T. Japanese population norms of EQ-5D-5L and health utilities index mark 3: disutility catalog by disease and symptom in community settings. Value Health 2021;24:1193–202. [DOI] [PubMed] [Google Scholar]
- 32. Göhler A, Geisler BP, Manne JM, Kosiborod M, Zhang Z, Weintraub WSet al. Utility estimates for decision-analytic modeling in chronic heart failure–health states based on New York Heart Association classes and number of rehospitalizations. Value Health 2009;12:185–7. [DOI] [PubMed] [Google Scholar]
- 33. Medtronic . Product specification of AZURE™ XT DR. https://www.medtronic.com/content/dam/medtronic-com/01_crhf/brady/pdfs/azure-xt-dr-mri-surescan-spec-sheet.pdf (25 July 2022, date last accessed).
- 34. Medtronic . Product specification of Cobalt™ HF CRT-D. http://www.medtronic.me/content/dam/medtronic-com/01_crhf/hf/spec-sheets/cobalt-crome/product-spec-sheet-cobalt-hf-crt-d-mri-suresacn-dtpb2d4.pdf (25 July 2022, date last accessed).
- 35. Toyoda K, Yoshimura S, Nakai M, Koga M, Sasahara Y, Sonoda Ket al. Twenty-Year change in severity and outcome of ischemic and hemorrhagic strokes. JAMA Neurol 2022;79:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ministry of Health, Labour and Welfare . Japanese life table in 2020. (in Japanese). https://www.mhlw.go.jp/english/database/db-hw/lifetb23nd/dl/tables.pdf.
- 37. Connolly SJ, Kerr CR, Gent M, Roberts RS, Yusuf S, Gillis AMet al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med 2000;342:1385–91. [DOI] [PubMed] [Google Scholar]
- 38. Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi Fet al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol 2009;20:241–8. [DOI] [PubMed] [Google Scholar]
- 39. Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee Ret al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002;346:1854–62. [DOI] [PubMed] [Google Scholar]
- 40. Yokoshiki H, Shimizu A, Mitsuhashi T, Ishibashi K, Kabutoya T, Yoshiga Yet al. Trends in the use of implantable cardioverter-defibrillator and cardiac resynchronization therapy device in advancing age: analysis of the Japan cardiac device treatment registry database. J Arrhythm 2020;36:737–45. Published 2020 Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Medical Economics Division, Health Insurance Bureau, Ministry of Health, Labour and Welfare, Full scale introduction of cost-effectiveness evaluations in Japan overview. https://c2h.niph.go.jp/tools/system/overview_en.pdf (20 February 2019, date last accessed).
- 42. Al Halabi S, Qintar M, Hussein A, Alraies MC, Jones DG, Wong Tet al. Catheter ablation for atrial fibrillation in heart failure patients: a meta-analysis of randomized controlled trials. JACC Clin Electrophysiol 2015;1:200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 44. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy Det al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637–44. [DOI] [PubMed] [Google Scholar]
- 45. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens Let al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 46. Anselmino M, Matta M, D'Ascenzo F, Bunch TJ, Schilling RJ, Hunter RJet al. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2014;7:1011–8. [DOI] [PubMed] [Google Scholar]
- 47. Yokoyama Y, Miyamoto K, Nakai M, Sumita Y, Ueda N, Nakajima Ket al. Complications associated with catheter ablation in patients with atrial fibrillation: a report from the JROAD-DPC study. J Am Heart Assoc 2021;10:e019701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wasmer K, Mönnig G, Bittner A, Dechering D, Zellerhoff S, Milberg Pet al. Incidence, characteristics, and outcome of left atrial tachycardias after circumferential antral ablation of atrial fibrillation. Heart Rhythm 2012;9:1660–6. [DOI] [PubMed] [Google Scholar]
- 49. Crossley GH, Padeletti L, Zweibel S, Hudnall JH, Zhang Y, Boriani G. Reactive atrial-based antitachycardia pacing therapy reduces atrial tachyarrhythmias. Pacing Clin Electrophysiol 2019;42:970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim D, Yang PS, Yu HT, Kim TH, Jang E, Sung JHet al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J 2019;40:2313–23. [DOI] [PubMed] [Google Scholar]
- 51. Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med 2013;158:338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jin MN, Kim TH, Kang KW, Yu HT, Uhm JS, Joung Bet al. Atrial fibrillation catheter ablation improves 1-year follow-up cognitive function, especially in patients with impaired cognitive function. Circ Arrhythm Electrophysiol 2019;12:e007197. [DOI] [PubMed] [Google Scholar]
- 53. Rinfret S, Cohen DJ, Lamas GA, Fleischmann KE, Weinstein MC, Orav Jet al. Cost-effectiveness of dual-chamber pacing compared with ventricular pacing for sinus node dysfunction. Circulation 2005;111:165–72. [DOI] [PubMed] [Google Scholar]
- 54. Edwards SJ, Karner C, Trevor N, Wakefield V, Salih F. Dual-chamber pacemakers for treating symptomatic bradycardia due to sick sinus syndrome without atrioventricular block: a systematic review and economic evaluation. Health Technol Assess 2015;19:1–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mar PL, Chen G, Gandhi G, Tang ZZ, Leiserowitz A, Tripuraneni Aet al. Cost-effectiveness analysis of magnetic resonance imaging-conditional pacemaker implantation: insights from a multicenter study and implications in the current era. Heart Rhythm 2018;15:1690–7. [DOI] [PubMed] [Google Scholar]
- 56. Gold MR, Padhiar A, Mealing S, Sidhu MK, Tsintzos SI, Abraham WT. Economic value and cost-effectiveness of cardiac resynchronization therapy among patients with mild heart failure: projections from the REVERSE long-term follow-up. JACC Heart Fail 2017;5:204–12. [DOI] [PubMed] [Google Scholar]
- 57. Noda T, Lu X, Ishiguro Y, Ikuemonisan J, Holbrook R, Tsintzos Set al. Cost-effective analysis of automated programming optimization in cardiac resynchronization therapy: Holistic Markov modelling. J Cardiol 2022;79:734–9. [DOI] [PubMed] [Google Scholar]
- 58. Simantirakis EN, Koutalas EP, Vardas PE. Arrhythmia-induced cardiomyopathies: the riddle of the chicken and the egg still unanswered? . Europace 2012;14:466–73. [DOI] [PubMed] [Google Scholar]
- 59. Stronati G, Guerra F, Urbinati A, Ciliberti G, Cipolletta L, Capucci A. Tachycardiomyopathy in patients without underlying structural heart disease. J Clin Med 2019;8:1411. Published 2019 Sep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The claims database that supports the findings of this study are available from Medical Data Vision Co., Ltd. but were used under licence for the current study; therefore, restrictions apply, and the data are not publicly available. For inquiries about access to the data set used in this study, please contact MDV (https://www.mdv.co.jp/; email address, ebm_sales@mdv.co.jp).
Other data underlying this article are available in the article and in its online Supplementary material.