Abstract
Background
Functionally disruptive variants in the glucokinase gene (GCK) cause a form of mild non-progressive hyperglycemia, which does not require pharmacological treatment. A substantial proportion of patients with type 2 diabetes (T2D) carry GCK variants. We aimed to investigate whether carriers of rare GCK variants diagnosed with T2D have a glycemic phenotype and treatment response consistent with GCK-diabetes.
Methods
Eight patients diagnosed with T2D from the Danish DD2 cohort who had previously undergone sequencing of GCK participated. Clinical examinations at baseline included an oral glucose tolerance test and continuous glucose monitoring. Carriers with a glycemic phenotype consistent with GCK-diabetes took part in a three-month treatment withdrawal.
Results
Carriers of pathogenic and likely pathogenic variants had lower median fasting glucose and C-peptide levels compared to carriers of variants of uncertain significance and benign variants (median fasting glucose: 7.3 (interquartile range: 0.4) mmol/l vs. 9.5 (1.6) mmol/l, p = 0.04; median fasting C-peptide 902 (85) pmol/l vs. 1535 (295) pmol/l, p = 0.03). Four participants who discontinued metformin treatment and one diet-treated participant were reevaluated after three months. There was no deterioration of HbA1c or fasting glucose (median baseline HbA1c: 49 (3) vs. 51 (6) mmol/mol after three months, p = 0.4; median baseline fasting glucose: 7.3 (0.4) mmol/l vs. 7.0 (0.6) mmol/l after three months, p = 0.5). Participants did not consistently fulfill best practice guidelines for GCK screening nor clinical criteria for monogenic diabetes.
Discussion
Carriers of pathogenic or likely pathogenic GCK variants identified by unselected screening in T2D should be reported, as they have a glycemic phenotype and treatment response consistent with GCK-diabetes. Variants of uncertain significance should be interpreted with care. Systematic genetic screening of patients with common T2D receiving routine care can lead to the identification and precise care of patients with misclassified GCK-diabetes who are not identifiable through common genetic screening criteria.
Keywords: T2D (type 2 diabetes), MODY (maturity-onset diabetes of the young), Diagnosis, Treatment
1. Introduction
Identifying the subtype of monogenic diabetes caused by functionally disruptive variants in the glucokinase gene GCK also known as maturity-onset diabetes of the young (MODY) type 2 or GCK-MODY is a compelling example of the benefits of an accurate genetic diagnosis. Loss-of-function variants in GCK cause a glucose-sensing defect with lifelong mildly and stably elevated plasma glucose values [[1], [2], [3]]. Fasting plasma glucose is elevated above normal levels in 98% of patients with GCK-MODY, and plasma glucose increments during an oral glucose tolerance test (OGTT) are usually <3 mmol/l4. Glucose-lowering treatment is not indicated in GCK-MODY as patients very rarely develop diabetic complications [5,6] and glucose-lowering treatments do not improve hyperglycemia [7,8]. Therefore, these patients are at risk of overtreatment, including hypoglycemia, if the genetic cause of the disease is not diagnosed.
Best practice guidelines suggest screening for GCK variants in diabetes patients with persistent fasting hyperglycemia, stably and mildly elevated glycated hemoglobin A1c (HbA1c) levels, and modest glucose increments during an OGTT [9], while many referral criteria for genetic testing rely on a “classic triad” of features consisting of 1) a young age at diagnosis, 2) autosomal dominant inheritance, and 3) lack of distinguishing characteristics for other types of diabetes [10]. However, it is estimated that almost 80% of monogenic diabetes cases do not have a genetic diagnosis and are instead undiagnosed or classified as type 1 diabetes or type 2 diabetes (T2D) [11,12], and large case cohorts have shown that a substantial number of patients diagnosed with clinical T2D also carry potentially disease-causing variants in monogenic diabetes genes [[13], [14], [15], [16]]. In individuals diagnosed with T2D where a GCK variant is identified by genetic screening, the following possibilities for the impact of the variant on the phenotype must be considered. First, the person could carry a functional GCK variant and be phenotypically consistent with GCK-diabetes, and thus be misclassified as having T2D when they in fact have monogenic diabetes and should be treated accordingly. Second, the person could be an incidental carrier of a benign GCK variant without relevance for the patient's pathophysiology, and the initial diagnosis of T2D would therefore be correct. Finally, the person could be a carrier of a functional GCK variant while also having other complicating pathophysiological features such as insulin resistance and/or beta cell failure, which would lead to a phenotype inconsistent with GCK-diabetes. In this case, even if the person could not be treated according to their genotype, the knowledge that they carried a variant relevant to the pathophysiology could have implications for monitoring, and screening in family members.
In this study, we aimed to characterize to what extent genetic analyses performed in the Danish DD2 cohort of patients with T2D could be translated to clinical care.
2. Materials and methods
2.1. Study cohort and genetic characterization
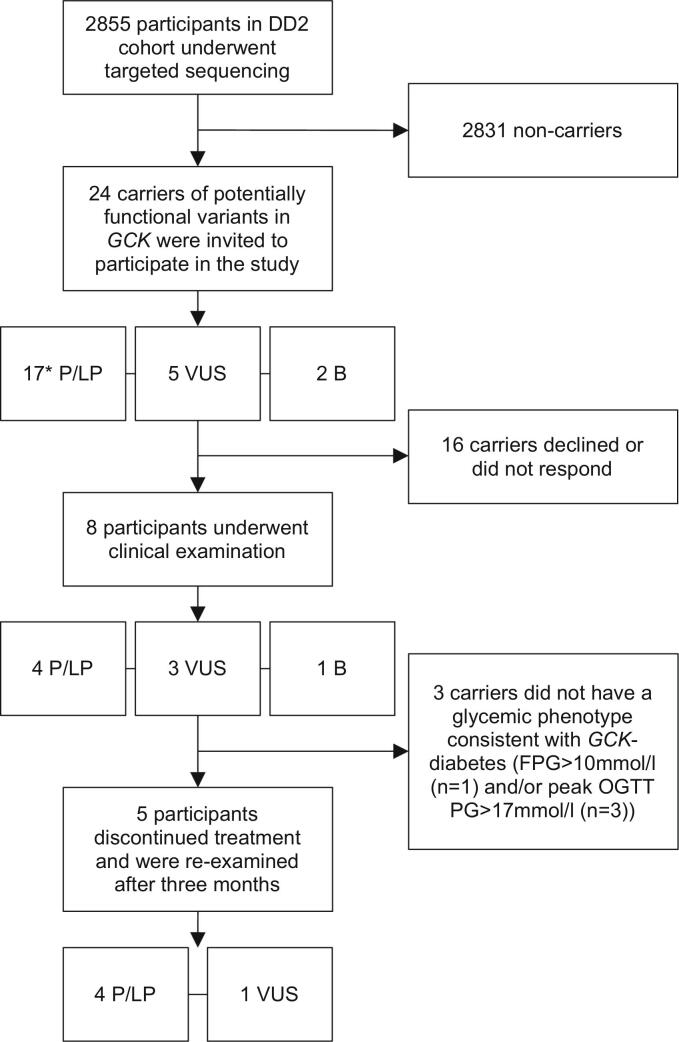
Participants from the nationwide Danish DD2 cohort [17] of newly diagnosed patients with T2D collected between 2010 and 2015 with available DNA samples (n = 2855) underwent targeted sequencing using a customized capture probe as previously described [18]. Variants were annotated according to RefSeq NM_000162. Carriers of potentially functional variants defined as nonsense, frame-shift, and missense variants, and variants up to two nucleotides into intron/exon bounds with a frequency < 0.1% in the Genome Aggregation Database [19] were eligible to take part in the study. For variants in ClinVar, the ClinVar classification was used, otherwise variants were manually classified as pathogenic (P), likely pathogenic (LP), variant of uncertain significance (VUS), likely benign (LB), or benign (B) according to the American College of Medical Genetics Standards and Guidelines [16,20]. Characteristics of carriers of P/LP variants at inclusion in DD2 have been published previously [16]. Twenty-four variant carriers (0.8% of the total cohort, see also Gjesing et al. [16]) were invited and eight participated between March 2020 and October 2021 (see Fig. 1). Non-participants were slightly older than participants (median participant age 64 years, median non-participant age 73 years), and among those that gave a reason for non-participation, the most commonly cited reason was lack of interest and inconvenience.
Fig. 1.
Flow chart of patient inclusion. Of 2855 participants from the DD2 cohort, 24 were carriers of potentially functional GCK variants, defined as nonsense, frame-shift, or missense variants, and variants up to two nucleotides into intron/exon bounds with a frequency < 0.1% in GnomAD. Eight participants agreed to take part in a clinical examination, and five continued on to the treatment discontinuation trial. B = benign variant carrier, FPG = fasting plasma glucose, P/LP = pathogenic or likely pathogenic variant carrier, OGTT = oral glucose tolerance test, PG = plasma glucose, VUS=Variant of Uncertain Significance carrier. *One carrier of an LP variant was also a carrier of a VUS.
2.2. Clinical examination at baseline
Body weight, standing height, waist circumference (midpoint between iliac crest and lower rib), hip circumference, and seated blood pressure were measured.
Participants self-reported age at diabetes diagnosis, use of any medications, known presence of diabetic retinopathy or peripheral neuropathy (yes/no if participant reported attending regular fundoscopy and podiatric assessment, unknown if participant did not report regularly attending both), and family history of diabetes.
2.3. Biochemistry
HbA1c was measured in EDTA-stabilized full blood (Tosoh G8 HPLC analyzer, Tosoh, Tokyo, Japan). Fasting plasma (p)-glucose, p-insulin, p-C-peptide, p-total cholesterol, p-HDL-cholesterol, and p-triglycerides were measured (Roche/Hitachi Cobas 6000 analyzer, Roche Diagnostics, Basel, Switzerland), and p-LDL-cholesterol was calculated using the Friedewald formula [21]. A urine sample was collected for the measurement of urinary albumin/creatinine ratio (UACR) (Roche/Hitachi Cobas 6000 analyzer, Roche Diagnostics, Basel, Switzerland). Homeostatic model assessment (HOMA) was calculated as an estimate of insulin resistance [22].
2.4. Categorical variables
BMI was divided into categories as follows: BMI < 18.5 kg/m2 = underweight, BMI ≥ 18.5 and < 25 kg/m2 = normal weight, BMI ≥ 25 and < 30 kg/m2 = overweight, BMI ≥ 30 kg/m2 = obese. Hypertension was defined as current, self-reported use of antihypertensive medication and/or mean blood pressure > 140/90 mmHg. Dyslipidemia was defined as current, self-reported use of lipid-lowering medication and/or fasting plasma triglycerides >1.7 mmol/l and/or serum HDL <1.2 mmol/l in female participants or < 1.0 mmol/l in male participants and/or serum LDL >2.5 mmol/l.
2.5. Oral glucose tolerance test
Participants underwent a 75 g OGTT after an overnight fast. Any glucose-lowering drugs were not taken in the 24 h preceding the examination. Blood for p-glucose, p-insulin and p-C-peptide measurement was collected at timepoints −10, −5, 15, 30, 45, 60, 90, 120, 180 min relative to the beginning of glucose ingestion. Glucose increments were calculated as 1) mean fasting values to maximal value, and 2) mean fasting value to 2-h value. The insulinogenic index (IGI) [23] was calculated as a measure of beta cell function. Insulin sensitivity index (ISI) was calculated according to the Matsuda method [24].
2.6. Mean glucose and glycemic variability
Participants were equipped with a Freestyle Libre Pro (Abbott, Chicago, IL, USA) continuous glucose monitor (CGM) for up to 14 days. Mean interstitial glucose and glycemic variability as expressed by the coefficient of variance (CV) and mean amplitude of glycemic excursion (MAGE) [25] were calculated from all available data after the first 24 h.
2.7. Treatment response
The subjects participated in withdrawal of glucose-lowering treatment if glucose values were below 17 mmol/l during the OGTT and FPG below 10 mmol/l (mean fasting and peak glucose for GCK-diabetes + ∼ 50% [4]). All treatment with glucose-lowering drugs was ceased and the following glycemic parameters were evaluated after three months: HbA1c, fasting p-glucose, CGM-derived mean interstitial glucose, fasting insulin and C-peptide, and glycemic variability, as well as changes in body composition, blood pressure, and lipid metabolism.
2.8. Data analysis
All calculations were performed in R v4.1.2. Scripts are available on https://github.com/acthuesen/gck.
A priori power calculations showed that the study would need to include 16 carriers to show a difference of 0.5 HbA1c % after treatment discontinuation with 80% power [26]. Thus, with the included participants, the study had only ∼36% power.
At baseline, carriers of P/LP variants, which are highly likely to be disease-causing and considered reportable, were compared to the remaining participants (carriers of VUS/B variants). P-values for differences between groups were calculated as Wilcoxon rank sum test for continuous data and Fisher's exact test for count data. In the analysis of treatment response, each included individual served as their own control. P-values for within-individual differences were calculated as Wilcoxon signed rank test.
2.9. Ethical approval
All participants gave written informed consent. The study was approved by the Regional Ethical Committee of the Capital Region of Denmark (H-18051923) and carried out in accordance with the Declaration of Helsinki.
3. Results
3.1. Gene variant characteristics
Gene variant characteristics are shown in Table 1. Eight carriers of eight rare GCK variants (P/LP: n = 4, VUS: n = 3, B: n = 1) took part in the study (Table 1). The c.214G > C variation of the G72R variant and C220Y variant were novel, while the other variants had either been previously identified in patients with GCK-MODY [3] (one variant), found in GnomAD [19] (two variants), or both (two variants). The T342P variant has previously been described as a MODY variant [3] but has since been confirmed as not pathogenic [27].
Table 1.
GCK variants in this study. Protein and nucleotide effect are annotated according to RefSeq NM_000162. Position called with human reference genome hg19. Classification refers to the classification in ClinVar, if the variant is known, otherwise manually classified according to the ACMG Standards and Guidelines [20]. MAF is the frequency in GnomAD. MAF = Minor Allele Frequency in the Genome Aggregation Database [19], MODY = Maturity Onset Diabetes of the Young, VUS = Variant of Uncertain Significance.
| Protein Effect | Nucleotide Effect | Position | Classification | MAF | Previosuly seen in MODY? |
|---|---|---|---|---|---|
| p.D4N | c.10G > A | 7_44228543_C_T | VUS | 0.00003 | No |
| p.G72R | c.214G > A | 7_44192019_C_T | Pathogenic | 0.000004 | Yes [3] |
| p.G72R | c.214G > C | 7_44192019_C_G | Pathogenic | 0 | No |
| p.E120K | c.358G > A | 7_44191875_C_T | VUS | 0.000004 | No |
| p.C220Y | c.659G > A | 7_44189379_C_T | Likely Pathogenic | 0 | No |
| p.T228M | c.683C > T | 7_44187429_G_A | Pathogenic | 0.000004 | Yes [3] |
| p.G246A | c.737G > C | 7_44187375_C_G | VUS | 0 | Yes [30] |
| p.T342P | c.1024A > C | 7_44185325_T_G | Benign | 0.000008 | Previously [3,27] |
3.2. Clinical characteristics
Clinical characteristics of study participants stratified into carriers of P/LP variants and VUS/B variants are shown in Table 2. Median age at diagnosis was 34 years among carriers of P/LP variants, and 59 years among carriers of VUS/B variants (p = 0.08). A first degree relative with diabetes was reported in all P/LP carriers but only one VUS/B carrier (p = 0.1). Two participants (both carriers of P/LP variants) had a family history clearly indicative of autosomal dominant inheritance.
Table 2.
Clinical characteristics of study participants, stratified into carriers of P/LP variants and VUS/B variants. Continuous variables are given as median (IQR) whereas count variables are given as absolute counts. Dyslipidemia includes use of lipid-lowering medication and hypertension includes use of antihypertensive medication. Combination therapies encompass combinations of metformin or sulphonylurea, glucagon-like peptide 1 receptor agonists, and sodium-glucose cotransporter-2 –inhibitors. B = Benign, BMI = Body Mass Index, CI = confidence interval, CV = Coefficient of Variance, HDL = high-density lipoprotein, HOMA-IR = Homeostasis model assessment for insulin resistance, IGI = insulinogenic index, IQR = interquartile range, ISI = insulin sensitivity index, LDL = low-density lipoprotein, LP = likely pathogenic, MAGE = Mean Amplitude of Glycemic Excursion, SD = standard deviation, VUS = Variant of Uncertain Significance, WHR = waist-to-hip ratio, P = pathogenic. P-values were calculated using Wilcoxon rank sum test for continuous data, and Fisher's exact test for counts. IQR was calculated as Q3-Q1.
| P/LP carriers |
VUS/B carriers |
||||
|---|---|---|---|---|---|
| Mean (95%CI) |
Median (IQR) |
Mean (95%CI) |
Median (IQR) |
P-value | |
| Counts | Counts | ||||
| Demographic, disease, and anthropometric characteristics | |||||
| Sex (male/female) | 0/4 | 2/2 | 0.4 | ||
| Age /years | 49.8 (20.8–78.7) | 50.0 (30.8) | 68.0 (55.6–80.4) | 69.0 (11.5) | 0.3 |
| Age at diagnosis (years) | 35.3 (11.4–59.1) | 34 (18) | 57.5 (44.9–70.1) | 59 (12) | 0.08 |
| Diabetes duration (years) | 14.5 (2.8–26.2) | 12.5 (5) | 10.5 (9.6–11.4) | 10.5 (1) | 0.3 |
| First degree relative with diabetes (yes/no) | 4/0 | 1/3 | 0.1 | ||
| Current diabetes treatment (diet/ metformin monotherapy/ combination therapy/insulin) | 1/3/0/0 | 0/2/2/0 | 0.4 | ||
| Diabetic retinopathy or peripheral neuropathy (yes/no/unknown) | 0/2/2 | 0/3/1 | 1 | ||
| BMI (kg/m2) | 35.5 (17.6–53.3) | 35.0 (15.1) | 31.4 (19.2–43.6) | 29.5 (6.2) | 0.9 |
| BMI category (normal weight/overweight/obese) | 1/1/2 | 1/1/2 | 1 | ||
| WHR | 0.85 (0.60–1.11) | 0.79 (0.11) | 0.98 (0.84–1.13) | 1.01 (0.11) | 0.3 |
| Lipids and cardiovascular health | |||||
| Total cholesterol (mmol/l) | 4.6 (3.8–5.8) | 4.8 (0.5) | 4.3 (2.4–6.2) | 3.9 (0.8) | 0.7 |
| LDL cholesterol (mmol/l) | 2.7 (1.6–3.9) | 2.7 (0.7) | 2.5 (0.6–4.3) | 2.5 (0.7) | 0.9 |
| HDL cholesterol (mmol/l) | 1,4 (1.0–1.9) | 1.4 (0.3) | 1.0 (0.7–1.2) | 0.9 (0.1) | 0.06 |
| Dyslipidemia (yes/no) | 3/1 | 4/0 | 1 | ||
| Systolic blood pressure (mmHg) | 126 (103–148) | 124 (19) | 135 (118–152) | 136 (15) | 0.5 |
| Diastolic blood pressure (mmHg) | 80 (69–91) | 80 (9) | 84 (75–93) | 82 (4) | 0.7 |
| Hypertension (yes/no) | 1/3 | 3/1 | 0.5 | ||
| Urinary albumin/creatinine ratio | 10 (−7–27) | 5.5 (6.5) | 135 (−118–387) | 84.5 (169.5) | 0.06 |
| Glycemic characteristics | |||||
| Fasting glucose (mmol/l) | 7.1 (6.1–8.0) | 7.3 (0.4) | 9.5 (6.5–12.4) | 9.1 (1.6) | 0.04 |
| Fasting insulin (pmol/l) | 86 (37–135) | 75 (27) | 158 (49–266) | 141 (47) | 0.06 |
| Fasting C-peptide (pmol/l) | 859 (659–1059) | 902 (85) | 1625 (1079–2171) | 1535 (295) | 0.03 |
| HbA1c (mmol/mol) | 49 (43–55) | 49 (5) | 56 (42–69) | 56 (14) | 0.3 |
| HbA1c (%) | 6.6 (6.1–7.2) | 6.6 (0.4) | 7.3 (6.0–8.5) | 7.3 (1.3) | 0.3 |
| Glucose increment (0−2h) (mmol/l) | 3.1 (−1.9–8.0) | 3.9 (3.5) | 5.9 (3.3–8.5) | 6.0 (1.4) | 0.2 |
| Glucose increment (0-max) (mmol/l) | 5.0 (3.8–6.3) | 5.0 (1.3) | 8.0 (5.5–10.6) | 8.4 (1.4) | 0.03 |
| HOMA-IR | 3.9 (1.5–6.4) | 3.5 (1.4) | 9.0 (5.3–12.7) | 8.2 (2.1) | 0.03 |
| ISI | 2.2 (1.5–2.9) | 2.1 (0.5) | 1.5 (0.4–2.7) | 1.6 (0.5) | 0.2 |
| IGI | 121.3 (49.7–192.9) | 126.1 (31.4) | 89.5 (−114.8–293.8) | 33.2 (68.1) | 0.3 |
| Mean interstitial glucose (mmol/l) | 6.2 (5.5–7.0) | 6.2 (0.73) | 7.8 (5.7–9.9) | 7.8 (1.29) | 0.1 |
| Tissue glucose CV% | 18.1 (8.2–28.0) | 15.9 (3.8) | 20.4 (11.6–29.1) | 19.8 (5.1) | 0.5 |
| MAGE (mmol/l) | 2.3 (0.7–3.9) | 1.9 (0.6) | 3.4 (1.4–5.3) | 3.8 (1.1) | 0.5 |
The distribution of overweight and obese carriers was identical in the two groups (p = 1). The majority of carriers of VUS/B variants had dyslipidemia (100%) and hypertension (75%) compared to 25% and 75% of carriers of P/LP variants (p = 1 and p = 0.5).
Two carriers of VUS/B variants were treated with combinations of metformin or sulphonylurea, glucagon-like peptide 1 receptor agonists, and sodium-glucose cotransporter-2 -inhibitors, five participants were treated with metformin monotherapy (500 mg once daily n = 2, 1000 mg twice daily n = 3), and one P/LP carrier did not receive any medicinal treatment, but had undergone gastric bypass surgery more than a decade prior and was diet-treated. No participants were treated with insulin. Carriers of P/LP variants had statistically significantly lower FPG (median (IQR): 7.3 (0.4) vs. 9.5 (0.4) mmol/l, p = 0.04), fasting C-peptide (median (IQR): 902 (85) vs. 1535 (295) pmol/l, p = 0.03). Similarly, P/LP carriers were less insulin resistant as expressed by HOMA-IR (median (IQR): 3.5 (1.4) vs 8.2 (2.1), p = 0.03). During the OGTT, P/LP carriers had lower maximal glucose increments (median (IQR): 5.0 (1.3) vs. 8.4 (1.4) mmol/l, p = 0.03). Two P/LP carriers had fasting to 2-h increments <3 mmol/l and two had increments <6 mmol/l. Results were similar when removing the participant who had previously undergone gastric bypass surgery and was diet-treated from the summary statistics.
3.3. Effects of treatment discontinuation
All participants with P/LP variants and one carrier of the VUS E120K had fasting p-glucose <10 mmol/l and maximal p-glucose during the OGTT <17 mmol/l. Four metformin-treated participants were asked to discontinue metformin treatment. One participant who had previously undergone gastric bypass surgery and was diet-treated was asked to disregard dietary restrictions. After three months, the degree of hyperglycemia was largely identical to that at baseline as expressed by fasting glucose and HbA1c (median (IQR) fasting glucose at baseline: 7.3 (0.4) vs. 7.0 (0.6) mmol/l after three months; median (IQR) HbA1c at baseline: 49 (3) vs. 51 (6) mmol/mol after three months)(see Table 3), and both were within acceptable glycemic ranges for both T2D [28] and GCK-MODY [29]. Fasting insulin and fasting C-peptide levels were non-significantly higher at three months compared to baseline, largely driven by one participant. Similarly, changes in weight, blood lipids, and blood pressure were minor and not statistically significant. As a sensitivity analysis, we removed the participant who had undergone gastric bypass surgery and was diet-treated, which did not alter the results.
Table 3.
Clinical characteristics of the five participants (all four carriers of P/LP variants and the carrier of the VUS E120K), four of which discontinued metformin treatment (500 mg once daily in two participants, 1000 mg twice daily in two participants) and one who was diet-treated at baseline, after three months, and difference. All data are given as median (IQR). The column difference refers to the summary of within-individual differences. BMI = body mass index, CI, confidence interval, CV = coefficient of variance, HDL = high-density lipoprotein, HOMA-IR = Homeostasis model assessment for insulin resistance, IGI = insulinogenic index, IQR = interquartile range, ISI = insulin sensitivity index, LDL = low-density lipoprotein, MAGE = mean amplitude of glycemic excursion, WHR = waist-to-hip ratio. P-values were calculated using Wilcoxon signed rank test. IQR was calculated as Q3-Q1.
| Baseline |
After three months without treatment |
Difference |
|||||
|---|---|---|---|---|---|---|---|
| Mean (95%CI) | Median (IQR) | Mean (95%CI) | Median (IQR) | Mean (95%CI) | Median (IQR) | P-value | |
| 33.3 (19.7–46.8) | 29.1 (16.5) | 33.6 (20.7–46.5) | 30.0 (14.9) | 0.4 (−0.6–1.3) | 0.06 (1.15) | 0.6 | |
| WHR | 0.85 (0.68–1.02) | 0.81 (0.08) | 0.87 (0.71–1.04) | 0.87 (0.18) | 0.02 (−0.08–0.12) | 0.01 (0.05) | 1 |
| Total cholesterol (mmol/l) | 4.4 (3.5–5.4) | 4.8 (1) | 4.3 (2.8–5.8) | 4.2 (1.6) | −0.1 (−1.5–1.2) | −0.2 (0.8) | 1 |
| LDL cholesterol (mmol/l) | 2.7 (1.9–3.5) | 2.5 (0.4) | 2.6 (1.5–3.7) | 2.7 (1.6) | −0.1 (−1.2–1.0) | −0.2 (0.5) | 0.8 |
| HDL cholesterol (mmol/l) | 1.3 (0.9–1.8) | 1.3 (0.3) | 1.1 (0.6–1.5) | 1.1 (0.5) | −0.2 (−0.6–0.1) | −0.1 (0.1) | 0.06 |
| Systolic blood pressure (mmHg) | 129 (111–146) | 132 (25) | 125 (103–147) | 124 (15) | −4 (−16–9) | −5 (5) | 0.6 |
| Diastolic blood pressure (mmHg) | 80 (73–88) | 82 (7) | 76 (64–89) | 78 (10) | −4 (−13–5) | −5 (8) | 0.3 |
| Urinary albumin/creatinine ratio | 14 (−2–29) | 6 (21) | 31 (−36–99) | 7 (11) | 18 (−56–91) | −2 (14) | 0.9 |
| Fasting glucose (mmol/l) | 7.2 (6.5–7.9) | 7.3 (0.4) | 7.0 (6.1–8.0) | 7.0 (0.6) | −0.1 (−0.6–0.3) | −0.3 (0.2) | 0.5 |
| Fasting insulin (pmol/l) | 120 (21–219) | 82 (63) | 135 (−54–325) | 78 (38) | 15 (−84–115) | −7 (48) | 1 |
| Fasting C-peptide (pmol/l) | 1109 (401–1817) | 911 (66) | 1314 (44–2584) | 934 (219) | 205 (−368–778) | 42 (209) | 0.6 |
| HOMA-IR | 5.6 (0.7–10.6) | 3.8 (3.0) | 6.5 (−3.6–16.7) | 3.5 (1.9) | 0.9 (−4.6–6.5) | −0.3 (2.0) | 1 |
| HbA1c (mmol/mol) | 49 (45–53) | 49 (3) | 50 (46–55) | 51 (6) | 1.4 (−2.6–5.4) | 1 (4) | 0.4 |
| HbA1c (%) | 6.6 (6.2–7.0) | 6.6 (0.3) | 6.8 (6.3–7.2) | 6.8 (0.6) | 0.1 (−0.2–0.5) | 0.1 (0.4) | 0.4 |
| Mean interstitial glucose (mmol/l) | 6.2 (5.7–6.7) | 6.2 (0.7) | 6.3 (5.7–7.0) | 6.4 (0.5) | 0.1 (−0.6–0.8) | −0.15 (0.5) | 1 |
| Tissue glucose CV% | 17.4 (10.4–24.4) | 15.6 (1.7) | 19.2 (11.3–27.0) | 17.8 (2.2) | 1.8 (−1.1–4.7) | 1.7 (1.9) | 0.2 |
| MAGE (mmol/l) | 2.2 (1.0–3.3) | 1.8 (0.2) | 2.3 (1.1–3.4) | 1.9 (0.3) | 0.1 (0.0–0.2) | 0.1 (0.1) | 0.06 |
3.4. Variant effect by classification
Of the eight participants included in the study, five (63%) could discontinue treatment and were therefore considered to have a glycemic phenotype consistent with GCK-diabetes. This was the case for all carriers of P/LP variants (four P/LP carriers successfully discontinued treatment of four included P/LP carriers) and one carrier of a VUS (one VUS carrier successfully discontinued treatment of three included VUS carriers). The carrier of a benign variant was not consistent with GCK-diabetes.
4. Discussion
In this study, we characterized a sampling of carriers of rare GCK variants from a large Danish cohort of patients with T2D. We found that carriers of P/LP variants had milder diabetes than carriers of VUS/B variants. Furthermore, carriers of P/LP variants and one carrier of a VUS could discontinue treatment safely and without deterioration of glycemia over three months of observation. This indicates that patients with T2D carrying functional GCK variants have misclassified type 2 diabetes and could potentially benefit from being identified through expanded genetic screening.
Our findings emphasize the importance of understanding the functional consequence of identified variants. Eight GCK variant carriers were included in the study, including carriers of three VUS. The D4N variant is present in the pancreatic isoform exon 1a, which harbors very few known disease-causing variants [3]. Consistently, our data did not support the D4N variant as disease-causing. Similarly, the carrier of the VUS G246A [30] variant did not have GCK-like diabetes. In contrast, the carrier of the VUS E120K had stable and mild diabetes, consistent with GCK-diabetes. This emphasizes a key challenge in the interpretation of VUS, i.e. that the evidence on these variants is not sufficient to classify a variant as P/LP or B/LB. As new evidence emerges, VUS may be reclassified. As such, each VUS identified by genetic screening should be carefully and individually evaluated with consideration of the phenotype, and care should be taken if a choice is made to report a VUS to a referring clinician. In contrast, we find that all carriers of P/LP variants were consistent with GCK-diabetes, re-emphasizing that variants classified as P/LP should always be considered reportable. We also report two, to our knowledge, novel GCK variants; C220Y and the c.214G > C variation of the known G72R variant which has previously been found in MODY, both of which were consistent with GCK-causal variants.
While best practice guidelines recommend screening patients with mild diabetes for GCK variants [9], many referral criteria for genetic testing are still based on a triad of features associated with clinically-defined MODY [10]. Referral criteria or algorithms are designed to improve the likelihood of finding a true positive result to balance the costs of genetic testing [31]. As sequencing becomes more affordable [32,33] this balance may shift towards more testing. Scrutiny of genetic testing strategies is increasingly important as ∼0.6% of individuals with the diagnosis T2D carry P/LP variants in GCK [13,16], an amount that far exceeds previous estimates of the prevalence of GCK-MODY [11,12].
In this study, the “classic triad” of referral criteria for genetic screening were of limited use in distinguishing carriers of P/LP variants from carriers of VUS/B variants. While we did find differences in BMI and the prevalence of dyslipidemia and hypertension between groups, it is important to note that the age and sex distributions were different between P/LP and VUS/B carriers which is likely to contribute to these differences [[34], [35], [36]]. Overall, features associated with T2D such as overweight/obesity and metabolic syndrome were near-ubiquitous among both groups and the variation in the age of diagnosis was large. The Exeter Diabetes App MODY probability calculator [31], developed for the use in individuals with an age of diagnosis <35 years, was only applicable to two individuals in this study, one of which had a probability of 4.6% and one with a probability of 35.8%. This serves as a reminder that age at diagnosis in GCK-MODY is dependent on when a person's blood sugar is first measured [37], and that a high age of diagnosis or presence of features associated with T2D that are common in the general population should not preclude genetic testing, particularly in light of obesity becoming increasingly prevalent [38].
Five patients in this cohort had a glycemic phenotype consistent with GCK-diabetes based on HbA1c, fasting p-glucose, and CGM measurements, yet they did not fulfill the “classic triad” for clinically-defined MODY [10]. In addition, two carriers of P/LP variants had glucose increments exceeding the 4.6 mmol/l, which is the cut-off suggested by best practice guidelines [9]. The genetic diagnosis coupled with glycemic characterization in this study led to discontinuation of metformin in four participants, while the fifth did not receive pharmacological treatment at the outset but was diet-treated. We observed only minor changes in HbA1c after metformin discontinuation. Studies of metformin withdrawal in prediabetic subjects and normoglycemic, normal-weight subjects with polycystic ovarian syndrome have shown that fasting and 2-h glucose levels increase after metformin discontinuation and that the glucose disposal rate in a euglycemic hyperinsulinemic clamp decreases [39,40]. The observed change in HbA1c in this study is in line with changes seen in metformin treatment in prediabetic obese subjects [41] or non-diabetic individuals with coronary artery disease [42], and is well under the mean change in HbA1c seen with metformin treatment in T2D patients [43]. HbA1c was within reference range for patients with GCK-MODY both at baseline and after three months [29]. Given that neither the “classic triad” nor best practice guidelines would have identified all patients with a glycemic phenotype consistent with GCK-diabetes in the present study, genetic screening of routine clinical care patients with diabetes should be considered in order to capture misdiagnosed patients with GCK-diabetes, benefitting patients who receive a more precise diagnosis and may be able to discontinue unnecessary treatment. Indeed, treatment for diabetes and related risk factors has been found to decrease quality of life (QOL) [44], while patients with GCK-MODY have been found to have a higher QOL than patients with type 1 diabetes, regardless of whether they receive pharmacological treatment or not [45]. Furthermore, family members can be identified through screening cascades. Given the substantial cost of treating diabetes [46], decreased medicalization of these patients also holds potential societal gains.
Many clinical factors should be considered in the decision to discontinue treatment. Importantly, GCK variants do not protect against other physiological states which may contribute to hyperglycemia e.g. insulin resistance and/or beta cell failure [47,48]. While we observed that carriers of P/LP variants were generally less insulin resistant than carriers of VUS/B variants, the average ISI and HOMA-IR was still in the insulin resistant range [24]. The follow-up time in this study is short, and as such, the long-term clinical trajectory of these patients is not known. In this respect, it is important to view the genetic test—especially in an unselected patient—as an added piece of information that the clinician can use in their collective assessment of a patient and not a final diagnosis. Factors such as insulin resistance, patient age, and comorbidities should factor into clinical decisions, also in carriers of P/LP variants. Similarly, given the increasing prevalence of obesity and T2D [38,49], combination of T2D and GCK-diabetes in the same patient may become more common in the future. It has been suggested that treatment should be initiated if HbA1c rises above 60 mmol/mol37. We show that glycemic variability as reflected in the OGTT increments and MAGE from the CGM are lower in carriers of P/LP variants than VUS/B carriers. Similarly, we have previously found that the variability in HbA1c measurements is lower in P/LP variants than in T2D patients [16]. More research is needed on whether e.g. glycemic variability could contribute to early identification of T2D in GCK-diabetes and how to best treat and monitor these patients.
While this study explores the opportunities provided by expanded genetic testing of unselected T2D patients, potential challenges related to genetic testing must be considered, including cost-benefit considerations [50] and—particularly if comprehensive analyses such as whole genome or exome sequencing are performed—data handling, management of incidental findings, as well as management of patient and clinician education and expectations [32,33].
The present study is limited by the low number of included participants, heterogeneity in treatment regimens prior to discontinuation, and lack of comparators. In particular, the study lacks statistical power and as such, the P-values presented in Table 2, Table 3 should be interpreted with care.
In conclusion, we showed that T2D carriers of GCK P/LP variants and the VUS E120K in this study had a glycemic phenotype consistent with GCK-diabetes and could discontinue treatment over three months of observation. Neither the conventional triad of features associated with monogenic diabetes nor best practice guidelines for GCK variant screening identified all patients, making a case for systematic use of genetic screening in patients with “common” T2D.
Funding information
This work was supported by grants from the Novo Nordisk Foundation (NNF17OC0028328) and the Novo Nordisk Foundation through the Challenge Program (NNFOC0033950). The Novo Nordisk Foundation Center for Basic Metabolic Research is supported by an unrestricted grant from the Novo Nordisk Foundation (NNF18CC0034900).
Disclosure summary
AT, RTJ, HM, MRK, HTS, AV, HBN, OBP, NG, JSJ, JR, APG, HS, TH: Nothing to disclose. TV has served on scientific advisory panels, been part of speaker's bureaus, served as a consultant to and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, GSK, Mundipharma, MSD/Merck, Novo Nordisk, Sanofi and Sun Pharmaceuticals.
CRediT authorship contribution statement
Anne Cathrine Baun Thuesen: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing. Rasmus Tanderup Jensen: Investigation, Writing – review & editing. Henrik Maagensen: Writing – review & editing. Maja Refshauge Kristiansen: Resources, Data curation, Writing – review & editing, Project administration. Henrik Toft Sørensen: Resources, Writing – review & editing. Allan Vaag: Resources, Writing – review & editing. Henning Beck-Nielsen: Resources, Writing – review & editing. Oluf B. Pedersen: Writing – review & editing. Niels Grarup: Writing – review & editing. Jens Steen Nielsen: Resources, Data curation, Writing – review & editing, Project administration. Jørgen Rungby: Resources, Writing – review & editing. Anette Prior Gjesing: Conceptualization, Methodology, Validation, Data curation, Writing – review & editing, Supervision, Project administration, Funding acquisition. Heidi Storgaard: Methodology, Validation, Writing – review & editing, Supervision, Project administration. Tina Vilsbøll: Methodology, Validation, Writing – review & editing, Supervision, Project administration, Funding acquisition. Torben Hansen: Conceptualization, Methodology, Validation, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Data availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1.Vionnet N., Stoffel M., Takeda J., Yasuda K., Bell G.I., Zouali H., Lesage S., Velho G., Iris F., Passa P., Froguel P., Cohen D. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature. 1992;356(6371):721–722. doi: 10.1038/356721a0. [DOI] [PubMed] [Google Scholar]
- 2.Hattersley A.T., Turner R.C., Patel P., O’Rahilly S., Hattersley A.T., Patel P., Wainscoat J.S., Permutt M.A., Tanazawa Y., Chin K.C., Watkins P. Linkage of type 2 diabetes to the glucokinase gene. Lancet. 1992;339(8805):1307–1310. doi: 10.1016/0140-6736(92)91958-B. [DOI] [PubMed] [Google Scholar]
- 3.Osbak K.K., Colclough K., Saint-Martin C., Beer N.L., Bellanné-Chantelot C., Ellard S., Gloyn A.L. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum. Mutat. 2009;30(11):1512–1526. doi: 10.1002/humu.21110. [DOI] [PubMed] [Google Scholar]
- 4.Stride A., Vaxillaire M., Tuomi T., Barbetti F., Njølstad P.R., Hansen T., Costa A., Conget I., Pedersen O., Søvik O., Lorini R., Groop L., Froguel P., Hattersley A.T. The genetic abnormality in the beta cell determines the response to an oral glucose load. Diabetologia. 2002;45(3):427–435. doi: 10.1007/s00125-001-0770-9. [DOI] [PubMed] [Google Scholar]
- 5.Steele A.M., Shields B.M., Wensley K.J., Colclough K., Ellard S., Hattersley A.T. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA. 2014;311(3):279–286. doi: 10.1001/jama.2013.283980. [DOI] [PubMed] [Google Scholar]
- 6.Pruhova S., Dusatkova P., Kraml P.J., Kulich M., Prochazkova Z., Broz J., Zikmund J., Cinek O., Andel M., Pedersen O., Hansen T., Lebl J. Chronic mild hyperglycemia in GCK-MODY patients does not increase carotid intima-media thickness. Int. J. Endocrinol. 2013 doi: 10.1155/2013/718254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stride A., Shields B., Gill-Carey O., Chakera A.J., Colclough K., Ellard S., Hattersley A.T. Cross-sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia. 2014;57(1):54–56. doi: 10.1007/s00125-013-3075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd M.H., Shields B.M., Hudson M., Pearson E.R., Hyde C., Ellard S., Hattersley A.T., Patel K.A. A UK nationwide prospective study of treatment change in MODY: genetic subtype and clinical characteristics predict optimal glycaemic control after discontinuing insulin and metformin. Diabetologia. 2018 doi: 10.1007/s00125-018-4728-6. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellard S., Bellanné-Chantelot C., Hattersley A.T. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51(4):546–553. doi: 10.1007/s00125-008-0942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peixoto-Barbosa R., Reis A.F., Giuffrida F.M.A. Update on clinical screening of maturity-onset diabetes of the young (MODY) Diabetology & Metabolic Syndrome. 2020;12(1):50. doi: 10.1186/s13098-020-00557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields B.M., Hicks S., Shepherd M.H., Colclough K., Hattersley A.T., Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53(12):2504–2508. doi: 10.1007/s00125-010-1799-4. [DOI] [PubMed] [Google Scholar]
- 12.Pang L., Colclough K.C., Shepherd M.H., McLean J., Pearson E.R., Ellard S., Hattersley A.T., Shields B.M. Improvements in awareness and testing Have led to a threefold increase over 10 years in the identification of monogenic diabetes in the U.K. Diabetes Care. 2022;45(3):642–649. doi: 10.2337/dc21-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnefond A., Boissel M., Bolze A., Durand E., Toussaint B., Vaillant E., Gaget S., Graeve F.D., Dechaume A., Allegaert F., Guilcher D.L., Yengo L., Dhennin V., Borys J.M., Lu J.T., Cirulli E.T., Elhanan G., Roussel R., Balkau B., Marre M., Franc S., Charpentier G., Vaxillaire M., Canouil M., Washington N.L., Grzymski J.J., Froguel P. Pathogenic variants in actionable MODY genes are associated with type 2 diabetes. Nature Metabolism. 2020;2(10):1126–1134. doi: 10.1038/s42255-020-00294-3. [DOI] [PubMed] [Google Scholar]
- 14.Flannick J., Beer N.L., Bick A.G., Agarwala V., Molnes J., Gupta N., Burtt N.P., Florez J.C., Meigs J.B., Taylor H., Lyssenko V., Irgens H., Fox E., Burslem F., Johansson S., Brosnan M.J., Trimmer J.K., Newton-Cheh C., Tuomi T., Molven A., Wilson J.G., O’Donnell C.J., Kathiresan S., Hirschhorn J.N., Njølstad P.R., Rolph T., Seidman J.G., Gabriel S., Cox D.R., Seidman C., Groop L., Altshuler D. Assessing the phenotypic effects in the general population of rare variants in genes for a dominant mendelian form of diabetes. Nat. Genet. 2013;45(11):1380–1385. doi: 10.1038/ng.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchsberger C., Flannick J., Teslovich T.M., Mahajan A., Agarwala V., Gaulton K.J., Ma C., Fontanillas P., Moutsianas L., McCarthy D.J., Rivas M.A., Perry J.R.B., Sim X., Blackwell T.W., Robertson N.R., Rayner N.W., Cingolani P., Locke A.E., Tajes J.F., Highland H.M., Dupuis J., Chines P.S., Lindgren C.M., Hartl C., Jackson A.U., Chen H., Huyghe J.R., van de Bunt M., Pearson R.D., Kumar A., Müller-Nurasyid M., Grarup N., Stringham H.M., Gamazon E.R., Lee J., Chen Y., Scott R.A., Below J.E., Chen P., Huang J., Go M.J., Stitzel M.L., Pasko D., Parker S.C.J., Varga T.V., Green T., Beer N.L., Day-Williams A.G., Ferreira T., Fingerlin T., Horikoshi M., Hu C., Huh I., Ikram M.K., Kim B.J., Kim Y., Kim Y.J., Kwon M.S., Lee J., Lee S., Lin K.H., Maxwell T.J., Nagai Y., Wang X., Welch R.P., Yoon J., Zhang W., Barzilai N., Voight B.F., Han B.G., Jenkinson C.P., Kuulasmaa T., Kuusisto J., Manning A., Ng M.C.Y., Palmer N.D., Balkau B., Stančáková A., Abboud H.E., Boeing H., Giedraitis V., Prabhakaran D., Gottesman O., Scott J., Carey J., Kwan P., Grant G., Smith J.D., Neale B.M., Purcell S., Butterworth A.S., Howson J.M.M., Lee H.M., Lu Y., Kwak S.H., Zhao W., Danesh J., Lam V.K.L., et al. The genetic architecture of type 2 diabetes. Nature. 2016;536(7614):41–47. doi: 10.1038/nature18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gjesing A.P., Engelbrechtsen L., Cathrine B., Thuesen A., Have C.T., Hollensted M., Grarup N., Linneberg A., Steen Nielsen J., Christensen L.B., Thomsen R.W., Johansson K.E., Cagiada M., Gersing S., Hartmann-Petersen R., Lindorff-Larsen K., Vaag A., Sørensen H.T., Brandslund I., Beck-Nielsen H., Pedersen O., Rungby J., Hansen T. 14-fold increased prevalence of rare glucokinase gene variant carriers in unselected Danish patients with newly diagnosed type 2 diabetes. Diabetes Res. Clin. Pract. 2022;194 doi: 10.1016/j.diabres.2022.110159. [DOI] [PubMed] [Google Scholar]
- 17.Sørensen H.T., Friborg S., Rungby J., Christensen J.S., Vaag A., Beck-Nielsen H. The Danish national type 2 diabetes cohort – the DD2 study. Clin Epidemiol. 2012;4(Suppl. 1):1–5. doi: 10.2147/CLEP.S31104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao R., Liu Y., Gjesing A.P., Hollensted M., Wan X., He S., Pedersen O., Yi X., Wang J., Hansen T. Evaluation of a target region capture sequencing platform using monogenic diabetes as a study-model. BMC Genet. 2014:15. doi: 10.1186/1471-2156-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., Gauthier L.D., Brand H., Solomonson M., Watts N.A., Rhodes D., Singer-Berk M., England E.M., Seaby E.G., Kosmicki J.A., Walters R.K., Tashman K., Farjoun Y., Banks E., Poterba T., Wang A., Seed C., Whiffin N., Chong J.X., Samocha K.E., Pierce-Hoffman E., Zappala Z., O’Donnell-Luria A.H., Minikel E.V., Weisburd B., Lek M., Ware J.S., Vittal C., Armean I.M., Bergelson L., Cibulskis K., Connolly K.M., Covarrubias M., Donnelly S., Ferriera S., Gabriel S., Gentry J., Gupta N., Jeandet T., Kaplan D., Llanwarne C., Munshi R., Novod S., Petrillo N., Roazen D., Ruano-Rubio V., Saltzman A., Schleicher M., Soto J., Tibbetts K., Tolonen C., Wade G., Talkowski M.E., Neale B.M., Daly M.J., MacArthur D.G. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., Voelkerding K., Rehm H.L. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics In Medicine. 2015;17:405. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 22.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Seltzer H.S., Allen E.W., Herron A.L., Brennan M.T. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus*. J. Clin. Invest. 1967;46(3):323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 25.Service FJ, Molnar G.D., Rosevear J.W., Ackerman E., Gatewood L.C., Taylor W.F. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 26.Jones B., Kenward M.G. Design and analysis of cross-over trials. Chapman and Hall/CRC. 2014 doi: 10.1201/b17537. [DOI] [Google Scholar]
- 27.Steele A.M., Tribble N.D., Caswell R., Wensley K.J., Hattersley A.T., Gloyn A.L., Ellard S. The previously reported T342P GCK missense variant is not a pathogenic mutation causing MODY. Diabetologia. 2011;54(8):2202–2205. doi: 10.1007/s00125-011-2194-5. [DOI] [PubMed] [Google Scholar]
- 28.Davies M.J., D’Alessio D.A., Fradkin J., Kernan W.N., Mathieu C., Mingrone G., Rossing P., Tsapas A., Wexler D.J., Buse J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD) Diabetologia. 2018;61(12):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 29.Steele A.M., Wensley K.J., Ellard S., Murphy R., Shepherd M., Colclough K., Hattersley A.T., Shields B.M. Use of HbA1c in the identification of patients with Hyperglycaemia caused by a Glucokinase mutation: observational case control studies. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haliloglu B., Hysenaj G., Atay Z., Guran T., Abalı S., Turan S., Bereket A., Ellard S. GCK gene mutations are a common cause of childhood-onset MODY (maturity-onset diabetes of the young) in Turkey. Clin. Endocrinol. 2016;85(3):393–399. doi: 10.1111/cen.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shields B.M., McDonald T.J., Ellard S., Campbell M.J., Hyde C., Hattersley A.T. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia. 2012;55(5):1265–1272. doi: 10.1007/s00125-011-2418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansen Taber K.A., Dickinson B.D., Wilson M. The promise and challenges of next-generation genome sequencing for clinical care. JAMA Intern. Med. 2014;174(2):280. doi: 10.1001/jamainternmed.2013.12048. [DOI] [PubMed] [Google Scholar]
- 33.Ormond K.E., Wheeler M.T., Hudgins L., Klein T.E., Butte A.J., Altman R.B., Ashley E.A., Greely H.T. Challenges in the clinical application of whole-genome sequencing. Lancet. 2010;375(9727):1749–1751. doi: 10.1016/S0140-6736(10)60599-5. [DOI] [PubMed] [Google Scholar]
- 34.Costanza M.C., Cayanis E., Ross B.M., Flaherty M.S., Alvin G.B., Das K., Morabia A. Relative contributions of genes, environment, and interactions to blood lipid concentrations in a general adult population. Am. J. Epidemiol. 2005;161(8):714–724. doi: 10.1093/aje/kwi103. [DOI] [PubMed] [Google Scholar]
- 35.Franklin S.S. Ageing and hypertension: the assessment of blood pressure indices in predicting coronary heart disease. J. Hypertens. Suppl. 1999;17(5):S29–S36. [PubMed] [Google Scholar]
- 36.Peters S.A.E., Muntner P., Woodward M. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the United States, 2001 to 2016. Circulation. 2019;139(8):1025–1035. doi: 10.1161/CIRCULATIONAHA.118.035550. [DOI] [PubMed] [Google Scholar]
- 37.Chakera A.J., Steele A.M., Gloyn A.L., Shepherd M.H., Shields B., Ellard S., Hattersley A.T. Recognition and management of individuals with hyperglycemia because of a heterozygous glucokinase mutation. Diabetes Care. 2015;38(7):1383–1392. doi: 10.2337/dc14-2769. [DOI] [PubMed] [Google Scholar]
- 38.Finucane M.M., Stevens G.A., Cowan M., Danaei G., Lin J.K., Paciorek C.J., Singh G.M., Gutierrez H.R., Lu Y., Bahalim A.N., Farzadfar F., Riley L.M., Ezzati M. National, regional, and global trends in body mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care. 2003;26(4):977–980. doi: 10.2337/diacare.26.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palomba S., Falbo A., Russo T., Manguso F., Tolino A., Zullo F., De Feo P., Orio F., Jr. Insulin sensitivity after metformin suspension in Normal-weight women with polycystic ovary syndrome. The Journal of Clinical Endocrinology & Metabolism. 2007;92(8):3128–3135. doi: 10.1210/jc.2007-0441. [DOI] [PubMed] [Google Scholar]
- 41.Aroda V.R., Knowler W.C., Crandall J.P., Perreault L., Edelstein S.L., Jeffries S.L., Molitch M.E., Pi-Sunyer X., Darwin C., Heckman-Stoddard B.M., Temprosa M., Kahn S.E., Nathan D.M., The Diabetes Prevention Program Research Group Metformin for diabetes prevention: insights gained from the diabetes prevention program/diabetes prevention program outcomes study. Diabetologia. 2017;60(9):1601–1611. doi: 10.1007/s00125-017-4361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preiss D., Lloyd S.M., Ford I., McMurray J.J., Holman R.R., Welsh P., Fisher M., Packard C.J., Sattar N. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. The Lancet Diabetes & Endocrinology. 2014;2(2):116–124. doi: 10.1016/S2213-8587(13)70152-9. [DOI] [PubMed] [Google Scholar]
- 43.Hirst J.A., Farmer A.J., Ali R., Roberts N.W., Stevens R.J. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012;35(2):446–454. doi: 10.2337/dc11-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang E.S., Brown S.E.S., Ewigman B.G., Foley E.C., Meltzer D.O. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30(10):2478–2483. doi: 10.2337/dc07-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szopa M., Matejko B., Ucieklak D., Uchman A., Hohendorff J., Mrozińska S., Głodzik W., Zapała B., Płatek T., Solecka I., Sani C.M., Małecki M.T. Quality of life assessment in patients with HNF1A-MODY and GCK-MODY. Endocrine. 2019;64(2):246–253. doi: 10.1007/s12020-018-1812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sortsø C., Green A., Jensen P.B., Emneus M. Societal costs of diabetes mellitus in Denmark. Diabet. Med. 2016;33(7):877–885. doi: 10.1111/dme.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fendler W., Małachowska B., Baranowska-Jazwiecka A., Borowiec M., Wyka K., Malecki M.T., Jarosz-Chobot P., Mysliwiec M., Mlynarski W., Group the PS Population-based estimates for double diabetes amongst people with glucokinase monogenic diabetes, GCK-MODY. Diabet. Med. 2014;31(7):881–883. doi: 10.1111/dme.12449. [DOI] [PubMed] [Google Scholar]
- 48.Martin D., Bellanné-Chantelot C., Deschamps I., Froguel P., Robert J.J., Velho G. Long-Term Follow-Up of Oral Glucose Tolerance Test–Derived Glucose Tolerance and Insulin Secretion and Insulin Sensitivity Indexes in Subjects With Glucokinase Mutations (MODY2) Diabetes Care. 2008;31(7):1321–1323. doi: 10.2337/dc07-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin X., Xu Y., Pan X., Xu J., Ding Y., Sun X., Song X., Ren Y., Shan P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci. Rep. 2020;10(1):14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naylor R.N., John P.M., Winn A.N., Carmody D., Greeley S.A.W., Philipson L.H., Bell G.I., Huang E.S. Cost-effectiveness of MODY genetic testing: translating genomic advances into practical health applications. Diabetes Care. 2014;37(1):202–209. doi: 10.2337/dc13-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.