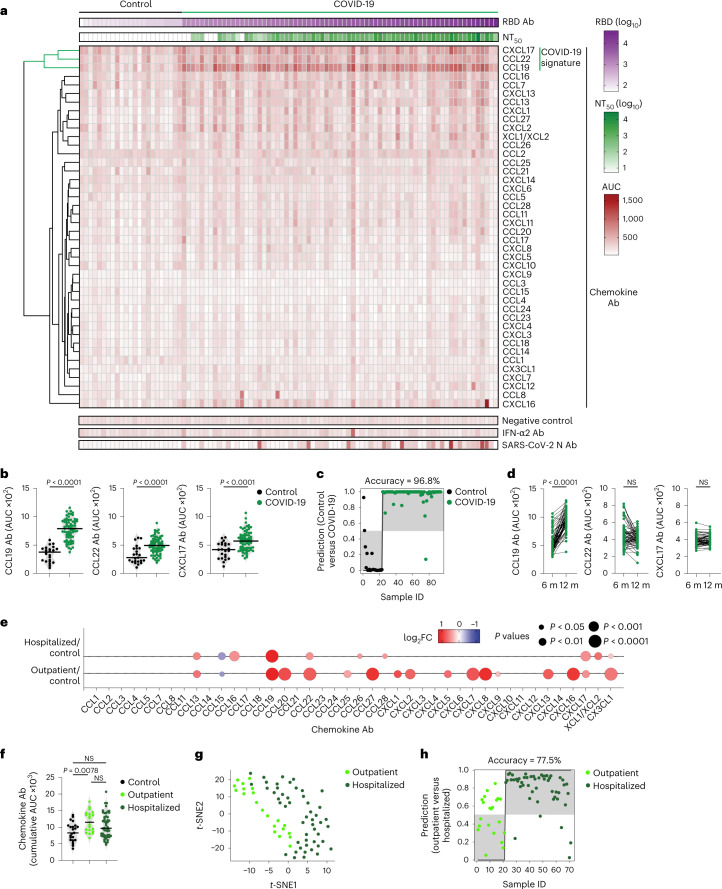
Fig. 1. Distinct patterns of chemokine antibodies in COVID-19 convalescents with different severity of acute disease.
a, Heatmap representing plasma IgG binding to 42 peptides comprising the N-loop of all 43 human chemokines, as determined by ELISA in healthy controls (Controls) and COVID-19 convalescents (COVID-19) of the Lugano cohort at month 6. Samples are ranked according to the level of SARS-CoV-2 RBD reactivity. Chemokine IgGs are ordered by unsupervised clustering analysis of ELISA signal. SARS-CoV-2 pseudovirus neutralizing activity (NT50) and IgG binding to peptides corresponding to negative control, IFN-α2 and SARS-CoV-2 nucleocapsid protein (N) are shown. b, AUC of ELISA showing IgG antibodies to CCL19, CCL22 and CXCL17 (COVID-19 signature) in healthy controls and COVID-19 convalescents at month 6. Two-tailed Mann–Whitney U-test. c, Logistic regression analysis showing the assignment of COVID-19 convalescents and healthy controls based on CCL19, CCL22 and CXCL17 antibodies at month 6. d, AUC of ELISA showing CCL19, CCL22 and CXCL17 antibodies at months 6 and 12 in COVID-19 convalescents (n = 63). Wilcoxon two-tailed signed-rank test. e, Chemokine antibodies in previously hospitalized and outpatient COVID-19 convalescents at month 6, shown as ratio over healthy controls. Circle size indicates significance; colors show the log2 fold-change increase (red) or decrease (blue), shown as ratio over healthy controls. Kruskal–Wallis test followed by Dunn’s multiple comparison test. f, Cumulative AUC of ELISA signal of the IgGs against the 42 chemokine N-loops in healthy controls and previously hospitalized and outpatient COVID-19 convalescents at month 6. Kruskal–Wallis test followed by Dunn’s multiple comparison test. g, t-SNE distribution of previously hospitalized and outpatient COVID-19 convalescents at month 6, as determined with the 42 datasets combined. h, Logistic regression analysis showing the assignment of previously hospitalized and outpatient COVID-19 convalescents based on CXCL5, CXCL8 and CCL25 antibodies (COVID-19 hospitalization signature) at month 6. In b and f, horizontal bars indicate median values. In a–h, AUC values are the average from two independent experiments. Healthy controls (n = 23) in a, b, c, e and f; COVID-19 convalescents (n = 71) in a, b and c, of which previously hospitalized (n = 50) and outpatient (n = 21) in e–h. Ab, antibody; AUC, area under the curve; FC, fold-change; ID, identity; m, months; NS, not significant.