Abstract
Genus Iris comprises numerous and diverse phytoconstituents displaying marked biological activities. The rhizomes, and aerial parts of Iris pseudacorus L. cultivars from Egypt and Japan were subjected to comparative metabolic profiling using UPLC-ESI-MS/MS. The antioxidant capacity was determined using DPPH assay. In vitro enzyme inhibition potential against α-glucosidase, tyrosinase and lipase was evaluated. In silico molecular docking was conducted on the active sites of human α-glucosidase and human pancreatic lipase. Forty-three compounds were tentatively identified including flavonoids, isoflavonoids, phenolics and xanthones. I. pseudacorus rhizomes extracts (IPR-J and IPR-E) exhibited the highest radical scavenging activity with IC50 values of 40.89 µg/mL and 97.97 µg/mL, respectively (Trolox IC50 value was 14.59 µg/mL). Moreover, IPR-J and IPR-E exhibited promising α-glucosidase inhibitory activity displaying IC50 values of 18.52 µg/mL, 57.89 µg/mL, respectively being more potent as compared to acarbose with IC50 value of 362.088 µg/mL. All extracts exerted significant lipase inhibitory activity exhibiting IC50 values of 2.35, 4.81, 2.22 and 0.42 µg/mL, respectively compared to cetilistat with IC50 value of 7.47 µg/mL. However, no tyrosinase inhibitory activity was observed for all I. pseudacorus extracts up to 500 µg/mL. In silico molecular modelling revealed that quercetin, galloyl glucose, and irilin D exhibited the highest fitting scores within the active sites of human α-glucosidase and pancreatic lipase. ADMET prediction (absorption, distribution, metabolism, excretion, and toxicity) showed that most of the phytoconstituents exhibited promising pharmacokinetic, pharmacodynamics and tolerable toxicity properties. According to our findings, I. pseudacorus might be considered as a valuable source for designing novel phytopharmaceuticals.
Subject terms: Secondary metabolism, Mass spectrometry, Metabolomics
Introduction
The prevalence of some chronic diseases including diabetes mellitus, hypertension, atherosclerosis, and others has rapidly expanded worldwide in the last decade. Therefore, there is a new global orientation for seeking effective and natural constituents for pharmaceutical industries1. Oxidative stress can be identified as the disruption in equilibrium between excessive production of oxidants throughout metabolism and presence of low levels of antioxidants within the body leading to cell and organ damage2. During the normal process of metabolism, the body releases free radicals at low to moderate concentrations3. However, owing to the prevalence of unhealthy diet, lifestyle, and environmental elements such as exposure to pollution and radiation, free radicals are released at high concentrations 3. Noteworthy, the occurrence of high levels of free radicals within the body leads to harmful alterations to cell constituents, such as proteins, lipids and DNA. Moreover, several pathological disorders result from this inverse modifications such as diabetes, hypertension, acute respiratory distress syndrome, atherosclerosis, chronic obstructive pulmonary disease, ischemia neurological disorders, and cancer2. Therefore, antioxidant agents have a vital role in keeping this balance3. There are numerous synthetic antioxidant agents that have been widely used in food and pharmaceutical industries such as butylated hydroxytoluene and butylated hydroxyanisole4. These synthetic antioxidants cause several adverse effects including increased risk of cancer, skin allergies and gastrointestinal tract problems4. Hence, natural antioxidant agents are generally more preferred nowadays4.
The predominance of some chronic illnesses including diabetes mellitus and obesity has rapidly increased implying a global health issue1. Different therapeutic approaches are frequently applied including key enzyme inhibitory theory that is considered as one of the most common strategies for controlling these ailments1. Several enzymes including α-glucosidase, lipase, and tyrosinase are considered as potential targets for lessening symptoms of diabetes mellitus, obesity and skin disorders, respectively1. Diabetes mellitus can be defined as a chronic metabolic ailment initiated by abnormal carbohydrate metabolism with a resultant hyperglycemic condition occurring from deficiency in insulin secretion, action, or both5. Therefore, the inhibition of α-glucosidase enzyme, a member of carbohydrate-digesting enzymes secreted in the small intestine of different organisms, delays glucose absorption and decreases the level of postprandial blood glucose. Hence, diabetes mellitus is controlled6. Noteworthy, α-glucosidase enzymes have an important role in the breakdown of complex carbohydrates releasing glucose into the small intestine. Glucose is absorbed in the blood circulation leading to increasing the postprandial hyperglycemia6. Synthetic α-glucosidase inhibitors that have been extensively used in pharmacy for the management of type II diabetic patients, such as acarbose, cause several side effects including flatulence, diarrhea, and abdominal distention4. Therefore, there is an increased desire for natural α-glucosidase inhibitory agents6.
Obesity is a worldwide epidemic chronic disease resulting from accumulation of excessive fat in adipose tissue and leading to complications as hypertension, heart disease, osteoarthritis in joints, hypercholesterolemia, cancer and diabetes mellitus7. The cornerstones in the management of obesity are supervised hypocaloric diet, physical exercise, pharmacotherapy and in the most critical cases, bariatric surgery8. Related to pharmacotherapy (anti-obesity treatment) is to block pancreatic lipase, a pancreatic enzyme that separates triglycerides into mono acyl glycerol and free fatty acids to ease their absorption7. There are many synthetic anti-obesity medications in the market, mainly, orlistat, but it is restricted due to its toxicity to various internal organs including the kidney and liver7. Therefore, there is an increased demand for natural-based drugs from plants that contain a substantial amount of lipase inhibitory compounds with minimal side effects.
The enzymatic browning and melanogenesis process that occur in different organisms are carried out by tyrosinase enzyme. Therefore, depigmentation agents are compounds that have the ability for tyrosinase inhibition. These agents are used widely in cosmetics and pharmaceutical formulations. Noteworthy, excessive melanin synthesis leads to various types of skin conditions including cervical poikiloderma, periorbital hyperpigmentation, skin cancer risk and Acanthosis nigricans9.
In continuation to our previous work on plants from family Iridaceae3,10–13, Iris pseudacorus L. was selected in the current study. Iris pseudacorus (common name: Yellow flag) is a perennial, monocotyledon, herbaceous and rhizomatous plant with yellow flowers14. It is native to Europe, Western Asia and North Africa15. Yellow flag grows in a variety of habitats, mostly preferring wetlands, riverbanks, places abundant with water15. Thus, its durability makes it a precious plant to consider for the use in wastewater treatment including the uptake of heavy metals, nitrogen and phosphorus wastes or pharmaceutical contaminants as codeine15. I. pseudacorus has displayed importance in folk medicine15. Infusions of the rhizomes were used by traditional Irish and British healers for the treatment of throat inflammations, colds, and toothache16. In addition, extracts of the plant were used to treat dandruff, wounds, and as excellent diuretic and tonic17. Moreover, the special prepared juice containing I. pseudacorus rhizome was used by English healers and administered every hour in syrup of buckthorn to treat dropsy when other medicines failed18. Tissues of I. pseudacorus accumulate various groups of biologically active substances including phenolic compounds, flavonoids, isoflavonoids, triterpenoids (iridals), organic acids, xanthones, anthocyanins, essential oil and others14. Extracts from I. pseudacorus could modulate osteoblasts and osteoclasts differentiation and hence, display anti-osteoporotic effects15. Besides, I. pseudacorus comprises compounds exhibiting estrogenic activity observed in both in vitro and in vivo studies15. Essential oils obtained from I. pseudacorus rhizomes possess antimicrobial activity against gram negative and positive pathogenic bacteria15. Polyphenols such as irilin B and trans-3-hydroxy-5,7-dimethoxyflavanone isolated from the roots inhibited the spontaneous colony formation and proliferation of colon carcinoma cells19. Iridals viz. isoiridogermananl and iridobelamal A isolated from rhizomes exhibited cytotoxic activity against five human tumor cell lines: HL-60, A-549, SMMC-7721, MCF-7 and SW-48015. To the best of our knowledge, no reports could be traced regarding the α-glucosidase, lipase and tyrosinase inhibitory activity of I. pseudacorus.
Therefore, the aim of the current study was to explore the secondary metabolites profile as well as assess the different biological activities of I. pseudacorus extracts. Herein, comparative metabolic profiling was achieved using ultra performance liquid chromatography coupled to electrospray ionization mass spectrometry (UPLC-ESI-MS/MS) analysis for the methanol extracts of I. pseudacorus rhizomes (IPR) and aerial parts (IPA) from Egypt and Japan. Moreover, the potential antioxidant and enzyme inhibitory activity of I. pseudacorus extracts on α-glucosidase, lipase and tyrosinase was evaluated in vitro. In addition, in silico docking studies were performed to validate the mechanism and binding pattern of the tentatively identified compounds to their targets. Besides ADMET prediction was performed to estimate pharmacokinetics, pharmacodynamics and toxicity properties of these compounds. Hence, the findings of the present study might enhance the knowledge regarding the therapeutic properties of I. pseudacorus.
Results
Liquid Chromatography Coupled to Mass Spectrometry (LC-MS) Phytochemical Profiles of Egyptian and Japanese I. pseudacorus Methanol Extracts
UPLC-ESI-MS/MS metabolite profiling of rhizomes and aerial parts of I. pseudacorus from Egypt and Japan revealed richness in biologically active compounds. The base peak chromatograms of I. pseudacorus aerial parts and rhizomes from Japan and Egypt in both negative and positive ionization modes are displayed in Figs. 1, 2 and 3. The tentatively identified compounds are summarized in Table 1 and illustrated in Fig. 4. The number of identified compounds in each class and their distribution are displayed in Table 2. Forty-three compounds were tentatively identified based on comparison of mass spectral data with literature database20–24. Compounds belonged to various classes including phenolics, flavonoids, isoflavonoids and xanthones.
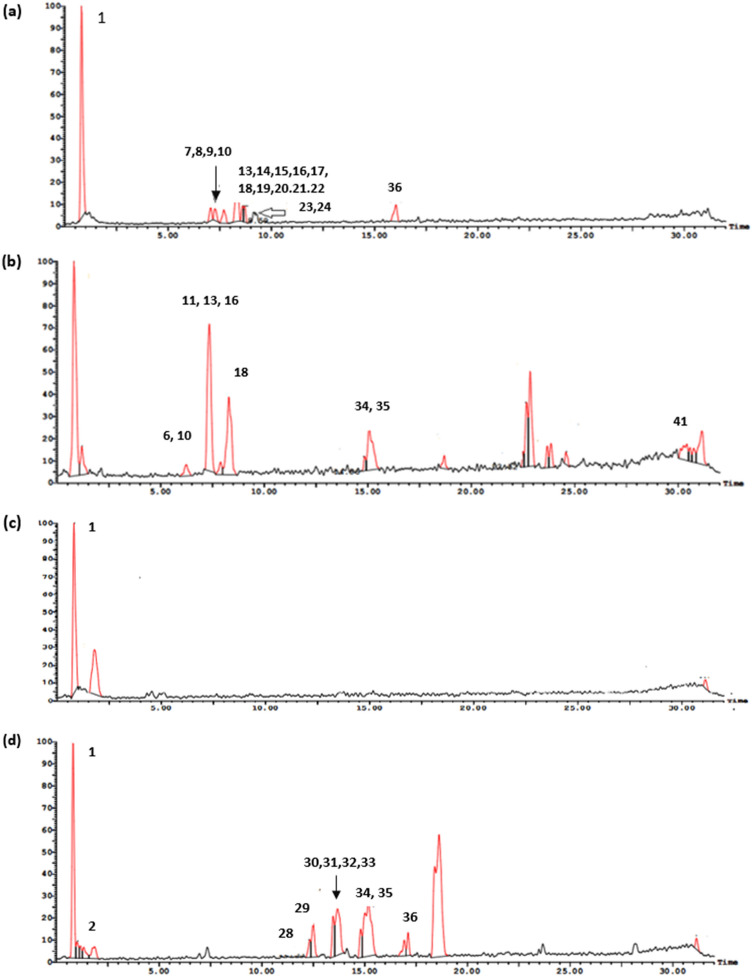
Figure 1.
LC/MS base peak chromatograms of IPA-J (a), IPA-E (b), IPR-J (c) and IPR-E (d) in negative ionization modes.
Figure 2.
LC/MS base peak chromatograms of IPA-J (a), IPA-E (b), IPR-J (c) and IPR-E (d) in positive ionization modes.
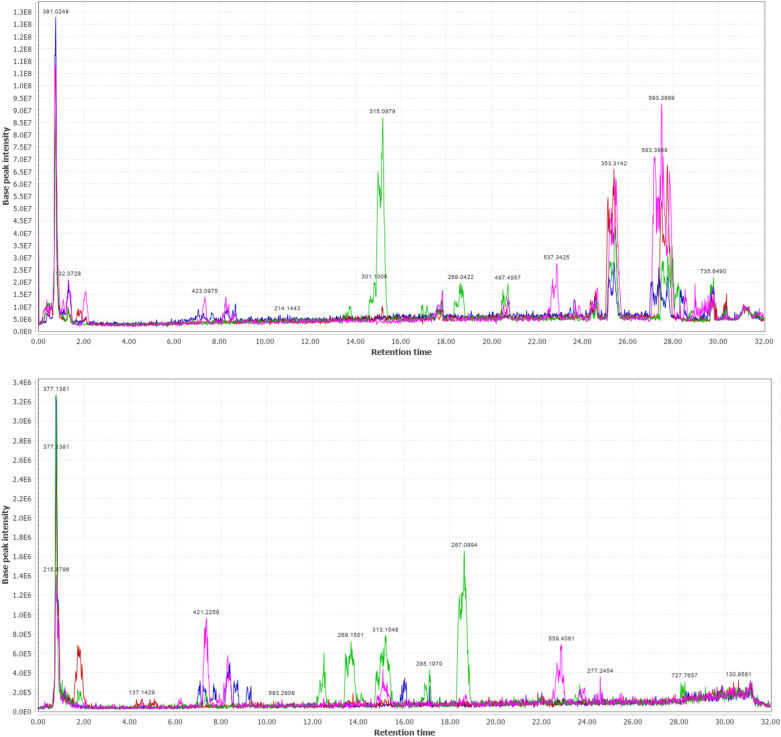
Figure 3.
The total ion chromatogram of the aerial parts and rhizomes extracts of I. pseudacorus in (a) positive, (b) negative ionization modes. IPA-J in blue, IPR-J in red, IPA-E in blue, IPR-E in green.
Table 1.
Secondary metabolites identified by UPLC-ESI-MS/MS analysis of I. pseudacorus extracts in both negative and positive ionization modes.
| No. | Rt (min) | [M-H]- | [M+H]+ | MSn ions (m/z) | Metabolite | Molecular formula | Class | References | IPA-J | IPA-E | IPR-J | IPR-E |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 0.66 | 341 | nd | 341: 179, 143, 125, 119, 101, 89, 71, 59. | O-Hexosyl-hexose (Sucrose) | C12H22O11 | Carbohydrates | 95,96 | + | - | + | + |
| 2. | 1.32 | 331 | nd | 331:169bp | O-galloyl hexose | C13H16O10 | Phenolic glycoside | 25 | - | - | - | + |
| 3. | 1.97 | nd | 166 | 166:120,103 | phenylalanine | C9H11NO2 | Amino acids | 97 | - | + | - | - |
| 4. | 2.78 | 341 | nd | 341:135 | Caffeic acid hexoside | C15H18O9 | Phenolic glycosides | 26 | + | - | - | - |
| 5. | 5.50 | 593 | nd | 593:407 | (epi) Gallocatechin-(epi)catechin dimer | C30H28O13 | Flavonoid | 4 | + | - | - | - |
| 6. | 6.03 | 583 | nd | 583:565,463,331, 301,259 | Neomangiferin | C25H28O16 | Xanthone | 67 | - | + | - | - |
| 7. | 6.88 | 593 | nd | 593:473,311,282 | Apigenin-C-hexoside-O-hexoside | C27H30O15 | Flavonoid | 39 | + | - | - | - |
| 8. | 6.88 | 593 | nd | 593:473,341,282 | Apigenin-C-hexoside-O-hexoside isomer | C27H30O15 | Flavonoid | 39 | + | - | - | - |
| 9. | 6.95 | 593 | nd | 593:473 BP, 503,311,282 | Apigenin-C-hexoside-O-hexoside (Saponarin) (Isovitexin-7-O-hexoside) | C27H30O15 | Flavonoid | 40 | + | - | - | - |
| 10. | 6.95 | 609 | nd | 609:327 bp | Luteolin-C-hexoside-O-hexoside | C26H28O16 | Flavonoid | 49 | + | + | - | - |
| 11. | 7.09, 7.15 | 421 | 423 | 421:331,301,271,259, 423:351,339,303,299,273,261 | Mangiferin | C19H18O11 | Xanthone | 67,68 | - | + | - | - |
| 12. | 7.15 | nd | 611 | 611:395,377,329,299,287 | Isoorientin-O-hexoside | C27H30O16 | Flavonoid | 46,48,50 | - | + | - | - |
| 13. | 7.21 | 421 | nd | 421:331,301,271,258 | Isomangiferin | C19H18O11 | Xanthone | 29 | + | + | - | - |
| 14. | 7.52 | 593 | nd | 593:341bp | Apigenin-C-hexoside-O-hexoside isomer (Isovitexin-O-hexoside) | C27H30O15 | Flavonoid | 40 | + | _ | _ | _ |
| 15. | 7.55 | 647 | nd | 647: 459 | Hydroxy-dimethoxy-flavone-acetyldeoxyhexosylhexoside | C31H36O15 | Flavonoid | 38 | + | _ | _ | _ |
| 16. | 7.55 | 609 | nd | 609:339,327 | Luteolin-C-hexoside-O-hexoside isomer | C26H28O16 | Flavonoid | 40 | + | + | - | - |
| 17. | 7.72 | 593 | nd | 593:399 | Luteolin-C-hexoside-C-deoxyhexoside | C27H30O15 | Flavonoid | 51 | + | - | - | - |
| 18. | 8.04 | 563 | nd | 563:443,407,383,353. | Apigenin-C-hexosyl-C- pentoside (Schaftoside) | C26H28O14 | Flavonoid | 41 | + | + | _ | _ |
| 19. | 8.14 | 447 | nd | 447:371,357,327,299,285,199 | Luteolin-C-hexoside (Orientin) | C21H19O11 | Flavonoid | 52,53 | + | - | _ | _ |
| 20. | 8.27 | 593 | nd | 593: 299,298 | Kaempferide hexosyl pentoside | C27H30O17 | Flavonoid | 32 | + | - | - | - |
| 21. | 8.27 | 593 | nd | 593: 357,285,284,150 | Kaempferol-O-deoxyhexosyl hexoside (Kaempferol-O-rutinoside) | C27H30O15 | Flavonoid | 32,33 | + | - | - | - |
| 22. | 8.41 | 593 | nd | 593: 473bp | Apigenin-di-C-hexoside (Vicenin-2) | C27H30O15 | Flavonoid | 42 | + | - | - | - |
| 23. | 9.29 | 447 | nd | 447:285 bp | Luteolin‐O‐ hexoside | C21H20O11 | Flavonoid | 53 | + | _ | _ | _ |
| 24. | 9.29 | 563 | nd | 563:311 | Apigenin-C-hexoside-O-pentoside | C26H28O16 | Flavonoid | 43,44 | + | - | - | - |
| 25. | 10.19 | 461 | nd | 461:299,283,255,240 | Dihydro-methoxyisoflavone-O-hexoside (Tectoridin) | C22H22O11 | Isoflavonoid | 56,57 | _ | _ | _ | + |
| 26. | 10.54 | 301 | nd | 301: 139bp | Hydroxy-methoxy-phenyl-O-hexoside (Tachioside) | C13H18O8 | Phenolic glycoside | 27 | - | - | - | + |
| 27. | 11.66 | 461 | nd | 461:284bp | Kaempferol-O-glucuronide | C21H18O12 | Flavonoid | 35 | _ | _ | _ | + |
| 28. | 12.24 | 315 | nd | 315: 300,272,227 | Tetrahydroxy-methoxyisoflavone (Irilin D) | C16H12O7 | Isoflavonoid | 29,58 | - | - | - | + |
| 29. | 12.64 | 299 | nd | 299:284bp,283,240 | Trihydroxy-methoxyisoflavone (Rhamnocitrin) | C16H12O6 | Flavonoid | 59 | - | - | - | + |
| 30. | 13.39 | 269 | nd | 269:241,225,201, 169,133 | Trihydroxyisoflavone (Genistein) | C15H10O5 | Isoflavonoid | 60 | - | - | - | + |
| 31. | 13.51 | 299 | 301 | 301:286,268,183,168,140. 299:284,212,200, 166. | Trihydroxy-methoxyisoflavone (Tectorigenin) | C16H12O6 | Isoflavonoid | 61–63 | - | - | - | + |
| 32. | 13.55 | 301 | nd | 301:286,164,151, 111,87 | Trihydroxy-methoxyflavanone (Hesperetin) | C16H14O6 | Flavonoid | 54,55,98 | _ | - | _ | + |
| 33. | 13.82 | 301 | nd | 301:283, 240, 151,139 | Dihydrokaempferide | C16H14O6 | Flavonoid | 29,30 | - | - | - | + |
| 34. | 14.72 | 313 | 315 | 315:300,272, 313:298,283,269, 252 | Dihydroxy Dimethoxy isoflavone (Irisolidone) | C17H14O6 | Isoflavonoid | 64,65 | - | + | - | + |
| 35. | 14.93 | 313 | 315 | 315: 300, 282, 269, 254, 183, 169, 168, 133, 313: 298, 267, 255,211, 183, 167. | Dihydroxy-dimethoxy-isoflavone isomer | C17H14O6 | Isoflavonoid | 55 | - | + | - | + |
| 36. | 16.43 | 329 | nd | 329: 229, 211, 171,139 | Trihydroxy-octadecenoic acid | C18H34O5 | Fatty acids | 53 | + | - | - | + |
| 37. | 21.18 | nd | 261 | 261: 243, 167, 121, 93 | Trihydroxy-methoxy-benzophenone | C14H12O5 | Benzophenone. | 69 | + | _ | _ | _ |
| 38. | 24.43 | nd | 303 | 303:303,285,267,257,165,153,95 | Pentahydroxy flavone (Quercetin) | C15H10O7 | Flavonoid | 28 | _ | + | + | + |
| 39. | 27.62 | nd | 317 | 317: 285,229,177,165, 153 | Tetrahydroxy-methoxyflavone (Isorhamnetin) | C16H12O7 | Flavonoid | 28 | + | - | + | + |
| 40. | 28.29 | nd | 433 | 433:271bp,227,145, 108 | Apigenin-O-hexoside | C21H20O10 | Flavonoid | 28,45 | _ | _ | + | _ |
| 41. | 30.11 | 317 | nd | 317: 271 | Trihydroxy-trimethoxy flavone (Myricetin) | C15H10O8 | Flavonoid | 36,37 | - | + | - | - |
| 42. | 30.27 | nd | 433 | 433:397 ,367 , 271, 309 ,342 | Apigenin-C-hexoside (Vitexin) | C21H20O10 | Flavonoid | 47,46 | _ | _ | + | _ |
| 43. | 31.38 | nd | 433 | 433:313,255, 121 | Apigenin-C-hexoside isomer (Isovitexin) | C21H20O10 | Flavonoid | 48 | _ | _ | + | _ |
Figure 4.
Representative compounds identified in I. pseudacorus methanol extracts using UPLC-ESI-MS/MS analysis in both ionization modes.
Table 2.
Tentatively identified compounds present in I. pseudacorus extracts.
| Samples | Number of detected compounds | |||||
|---|---|---|---|---|---|---|
| Flavonoids | Isoflavonoids | Xanthones | Phenols | Fatty acids | All | |
| IPA-J | 17 | 0 | 1 | 2 | 1 | 21 |
| IPR-J | 5 | 0 | 0 | 0 | 0 | 5 |
| IPA-E | 6 | 2 | 3 | 1 | 0 | 12 |
| IPR-E | 6 | 6 | 0 | 2 | 1 | 15 |
Phenolic acid Derivatives
Compound (2) displayed a pseudomolecular ion peak at m/z 331 [M-H]-. The MS2 profile presented a characteristic base peak at m/z 169 [M-H-162]- corresponding to the natural loss of a hexosyl residue25. Compound (2) was tentatively identified as O-galloyl hexose. Compound (4) showed a molecular ion peak [M-H]- at m/z 341. The MS2 spectrum displayed a characteristic base peak at m/z 135 [M-H-162-CO2]- corresponding to decarboxylated caffeic acid after elimination of both hexose and CO2 molecule26. Consequently, compound (4) was recognized as caffeic acid hexoside. Compound (26) exhibited a molecular ion peak at m/z 301[M-H]-. Its MS2 profile revealed a characteristic base peak at m/z 139 [M-H-162]- owing to the neutral loss of a hexosyl moiety27. Thus, compound (26) was putatively identified as hydroxy-methoxy-phenoxy-hexoside.
Flavonoids
Various flavonoids were previously detected and isolated from I. pseudacorus extracts either in aglycone or glycosylated forms including flavones, flavanones, flavanols, flavonols and isoflavonoids. Mass spectra can predict the skeleton of flavonoids via several fragmentation pathways28.
Flavanols
Compound (5) exhibited a molecular ion peak [M-H]- at m/z 593 and eluted at Rt 5.50 min. The MS2 spectrum displayed a characteristic base peak at m/z 407 [M-H-168-H2O]- was produced via RDA mechanism and successive loss of a water molecule4. This fragment is characteristic for (epi)gallocatechin. Thus, compound (5) was tentatively identified as proanthocyanidin dimer ((epi)gallocatechin-(epi)catechin dimer). Compound (33) revealed [M-H]- with value of m/z 301. Its MS2 spectrum exhibited typical fragment ion peaks at m/z 283 [M-H-H2O]- attributed to the loss of a H2O molecule, m/z 255 probably due to a subsequent loss of C2H4 moiety, and m/z 240 due to further loss of a methyl moiety. A fragment ion peak at m/z 151 was attributed to [1,3A-H]-29,30. Thus, compound (33) was tentatively identified as dihydrokaempferide and was previously isolated from the rhizomes of I. tectorum31.
Flavonols
Compound (20) demonstrated a molecular ion peak [M-H]- with m/z 593. Its MS2 profile exhibited the aglycone ion [Y°]- at m/z 299 and radical aglycone ion [Y°-H]- (base peak) at m/z 298 produced from homolytic and heterolytic cleavage, indicating that the kaempferide aglycone. Thus, compound (20) was putatively recognized as kaempferide hexosyl pentoside, which is reported for the first time in the genus Iris32. Compound (21) showed [M-H]- at m/z 593. Its MS2 spectrum revealed the aglycone ion [Y˚]- at m/z 285 and radical aglycone ion [Y˚-H]- (base peak) at m/z 284 corresponding to kaempferol aglycone as a result of the successive loss of hexose and deoxyhexose moieties, m/z 357 [M-H-146-90]- due to loss of deoxyhexose and cleavage of a hexosyl unit and m/z 150 relative to [1,3A--H]- RDA fragmentation. Thus, compound (21) was identified as kaempferol-O-deoxyhexosyl hexoside32,33. Kaempferol-3-O-rutinoside was previously isolated from the rhizomes of I. pseudopumila34. Compound (27) displayed a molecular ion peak [M-H]- at m/z 461. Its MS2 profile revealed distinctive radical aglycone ion for kaempferol (base peak) at m/z 284 [Y˚-H]– due to neutral loss of one glucuronyl unit35. Thus, compound (27) was tentatively identified a kaempferol-O-glucuronide. This is the first report of this compound in genus Iris according to our knowledge.
Compound (38) showed a pseudomolecular ion peak at m/z 303 [M+H]+ and was tentatively recognized as pentahydroxyflavone (quercetin). Its MS2 spectrum revealed distinguished fragment ion peaks at m/z 285 is due to [M+H- H2O]+, m/z 267 corresponding to [M+H-2H2O]+, m/z 257 owing to [M+H-H2O-CO]+, m/z 165, m/z 153 corresponding to [0,2A]+ and [1,3A]+ , respectively, arising from C-ring cleavage. And finally a fragment ion at m/z 95 due to [0,2B+–CO-O]+28. Quercetin was previously isolated from many species Iris including I. germanica34. Compound (39) showed a molecular ion peak [M+H]+ at m/z 317. Its MS2 profile exhibited characteristic fragment ion peaks at m/z 285 corresponding to [M+H-CH3-OH]+, m/z 229 corresponding to [M+H–CH3-OH-2CO]+, m/z 177 due to [M+H–2CO–C4H4O2]+, And finally fragment ion peaks at m/z 165 and 153 corresponding to [0,2A]+, [1,3A]+ fragments arising from C-ring cleavage28. Thus, compound (39) was tentatively identified as tetrahydroxy-methoxyflavone (isorhamnetin) and previously isolated from Iris pseudacorus34.Compound (41) eluted at Rt 30.11 min exhibited a molecular ion peak [M-H]- at m/z 317 and its distinctive fragment at m/z 271 due to loss of CO and H2O molecules36,37. Thus, compound (41) was tentatively identified as myricetin. It was previously isolated from I. sanguinea34.
Flavones
Compound (15) has molecular ion peak [M-H]- at m/z 647 was tentatively identified as hydroxy-dimethoxyflavone-acetyldeoxyhexosyl-hexoside. Its MS2 profile showed distinctive base peak at m/z 459 [M-H-146-42]- corresponding to loss of acetylrhamnose moiety from C-glycosylflavone38. 5-hydroxyl-4′,7-dimethoxyflavone-6-C-[O-(α-L-3′′′-acetylrhamnopyranosyl)-1→2-β-D-glucopyranoside was previously isolated from the leaves of I. tectorum Maxim38.
Apigenin derivatives
Among the identifed flavones: several apigenin derivatives were tentatively assigned in I. pseudacorus extracts. Compounds (7, 8, 9, 14) showed the same molecular ion peak [M-H]- at m/z 593 but eluted at different retention times. They were all tentatively identified as isomers of apigenin-C-hexoside-O-hexoside. These compounds displayed the common fragmentation pattern of C-glycosides. Compounds (7 and 8) exhibited characteristic fragment ion peaks at m/z 473 [M-H-120]− corresponding to 0,2X- internal cleavage of C-linked hexose, m/z 311 [M-H-120-162]– in compound (7) and m/z 341 [M-H-90-162]– in compound (8) owing to the neutral loss of a hexosyl moiety indicating the presence of a O-hexosyl unit. A fragment ion peak at m/z 282 was due to a subsequent loss of CHO moiety. Thus, compounds (7) and (8) were tentatively identified as isomers of apigenin-C-hexoside-O-hexoside39. Isovitexin-O-glucoside was previously isolated from many species of the genus Iris including I. setosa34. Compound (9) showed typical fragment ion peaks at m/z 503 [M-H- 0,3X]− and m/z 473 [(M-H)-0,2X]−. Other distinguishing fragment ion peaks at m/z 311 [M-H-120-162]– and m/z 282. Thus, compound (9) was tentatively identified as apigenin-C-hexoside-O-hexoside (isovitexin-7-O-glucoside)40. Compound (14) displayed distinctive base peak at m/z 341 [M-H-90-162]– and absence of other characteristic fragment ion peaks at m/z 503 [M-H-90]− and m/z 473 [M-H-0,2X]−. This indicated to the presence of a terminal O-hexosyl unit and not directly attached to the apigenin aglycone. Thus, compound (14) was tentatively identified as apigenin-C-hexoside-O-hexoside isomer (Isovitexin-X′′-O-glucoside)40. compound (9) and (14) were isolated from the aerial parts of I. ensata and I. sanguinea, respectively34.
Compound (18) exhibited a molecular ion peak [M-H]- at m/z 563. Its fragmentation pattern related to asymmetric di-C-glycosides. The MS2 profile showed characteristic fragment ion peaks at m/z 443 due to [M-H-0,2X]-, m/z 407 owing to [M-H-0,2X -2H2O]-, m/z 383 [M-H-0,2X –60]- and m/z 353 [M-H-0,2X –90]- is due to cleavage of C-pentosyl residue. Besides the absence of fragmentation ion peak for C-pentosyl unit at m/z 503 [M-H-60]-, suggested that the location of the hexose unit is at position 6. The ions at m/z 353 (AGly + 83) and m/z 383 (AGly + 113) are typical fragments of the di-C-glycosyl flavonoids further confirms the proposed structure. Thus, compound (18) was tentatively identified as apigenin-6-C-hexosyl-8-C-pentosyl (schaftoside)41. It was previously isolated from I. germanica34. Compound (22) showed a molecular ion peak [M-H] at m/z 593. The MS2 profile revealed the distinctive base peak at m/z 473 corresponding to [M-H-0,2X]. Thus, compound (22) was tentatively identified as apigenin di-C-hexoside (vicenin-2)42. It was previously reported in I. ensata34. Compound (24) displayed a molecular ion peak [M-H]– at m/z 563. The MS2 profile revealed the distinctive base peak at m/z 311 [M-H-120-132]- relative to 0,2X+ fragmentation of a C-hexosyl unit and loss of O-pentosyl unit. Presence of the base peak at m/z 311 (AGly + 41) indicating that the aglycone is apigenin43,44. Thus, compound (24) was tentatively identified as apigenin-C-hexoside-O-pentoside. Compound (40) showed a molecular ion peak [M+H]+ at m/z 433, and a base peak at m/z 271 [M+H-162]+ indicating to loss of one O-hexosyl unit yielding the corresponding apigenin aglycone45. A fragment ion at m/z 227 [M+H-162- CO2]+ was attributed to the loss of CO2 molecule and at m/z 145 corresponding to [0,4B–H2O]+28. Thus, compound (40) was tentatively identified as apigenin-O-hexoside. Apigenin-7-O-glucoside was previously isolated from I. sisyrinchium L.34. Compound (42) eluted at Rt 30.27 min showed a molecular ion peak [M+H]+ at m/z 433. The MS2 spectra exhibited characteristic fragment ion peaks at m/z 397 [M+H-2*H2O]+, m/z 367 [M+H-30-36]+ due to cleavage of a C-hexosyl unit and the loss of two H2O molecules, m/z 309 [M+H-96-28]+corresponding to [0,4X+-2H2O-CO], m/z 342 [M+H-90-H]+ due to 0,3X+ fragmentation in C- hexosyl unit and finally m/z 271 [M+H-162]+ giving the corresponding apigenin aglycone. Thus compound (42) was tentatively identified as apigenin-C-hexoside46,47. While Compound (43) eluted at Rt 31.38 min and exhibited the same molecular ion peak. The MS2 spectrum displayed characteristic fragment ion peaks at m/z 313 [M+H−120]+ due to 0,2X+ fragmentation of a C-hexosyl unit, m/z 255 [M+H−120-58]+ corresponding to 0,2X+−2CHO and m/z 121 due to 0,2B+ fragmentation arising from C-ring cleavage. Indicating that the B ring was monohydroxylated (apigenin derivatives). Thus, compound (43) was tentatively identified as apigenin-C-hexoside isomer48. Compounds (42) and (43) displayed the same molecular ion peak [M+H]+ at m/z 433 but eluted at different retention times. According to the order of elution as reported in literature46,48 vitexin elutes earlier than isovitexin. Thus, compound (42) was tentatively identified as vitexin and compound (43) as isovitexin. They were previously isolated from many species of genus Iris34.
Luteolin derivatives
Compound (10) showed a molecular ion peak [M-H]- at m/z 609 and a base peak appeared at m/z 327 [M-H-162-120]- attributed to the loss of an O-linked hexoside and 0,2X+ fragmentation of a C-hexosyl unit indicating a luteolin aglycone49. Thus, compound (10) was tentatively identified as luteolin-C-hexoside-O-hexoside. Isoorientin-X′′-O-glucopyranoside was previously reported in I. sanguinea34. Compound (12) showed a molecular ion peak [M+H]+ at m/z 611. The MS2 spectrum exhibited typical fragment ion peaks at m/z 395 [M+H−162-54]+ attributed to loss of O-hexosyl moiety and 3 H2O molecules, m/z 329 [M+H-120-162]+ and m/z 299 [M+H-150-162]+. This fragmentation pattern corresponded to 0,2X+ and 0,1X+ cleavage of a C-hexosyl unit in addition to the loss of O-hexosyl moiety. Another fragment ion peak at m/z 287 [M+H-90- CO-CO2]+ corresponded to [0,3X+-CO-CO2]. The base peak at m/z 329 (AGly + 41) indicating a luteolin aglycone. The high abundance of m/z 299 and absence of m/z 300 fragment ion indicated isoorientin (6-C-glycoside) instead of orientin (8-C-glycoside)46,48,50. Thus, compound (12) was tentatively identified as isoorientin-O-hexoside.
Compound (16) exhibited a molecular ion peak [M-H]- at m/z 609. The MS2 profile revealed a distinctive base peak at m/z 339 [M-H-270]- due to the loss of both 90 and 180 amu. A fragment ion peak at m/z 327 [M-H-162-120]-. The occurrence of a product ion at m/z 327 (AGly + 41) indicated a luteolin aglycone. Thus, compound (16) was tentatively identified as luteolin-C-hexoside-O-hexoside isomer40. Compound (17) exhibited a molecular ion peak [M-H]- at m/z 593. Its MS2 profile showed the distinctive base peak at m/z 399 [M-H-104-90]- attributed to the cleavage of a C- deoxyhexosyl and a C-hexosyl unit. This fragment ion is very characteristic for di-C-glycosyl flavonoids. The presence of a base peak at m/z 399 (AGly +113) indicated a luteolin aglycone. Thus, compound (17) was tentatively identified as luteolin-C-hexoside-C-deoxyhexoside51. It was reported for the first time in genus Iris to the best of our knowledge.
Compound (19) displayed a molecular ion peak [M-H]- at m/z 447. The base peak at m/z 327 due to [M-H-0,2X]- and a fragment ion at m/z 357 owing to [M-H-0,3X]–. Product ions at m/z 299 [M-H-0,2X--CO]- was detected to the subsequent loss of a carbonyl group, and m/z 285 corresponding to [M-H-0,3X--CO-CO2]- 52. The differentiation between isoorientin and orientin was made considering the MS2 fragment ions at m/z 429 [M–H–H2O]− and m/z 411 [M–H–2*H2O]−, which are characteristic for isoorientin and are absent in orientin. In addition, the base peaks at m/z 357 and m/z 327 were characteristic for isoorientin and orientin, respectively53. Thus, compound (19) was identified as luteolin-8-C-hexoside (orientin). It was previously reported in many species of genus Iris34.Compound (23) exhibited a molecular ion peak [M-H]- at m/z 447 and strong fragment ion at m/z 285 [M-H-162]– corresponding to the loss of a hexosyl moiety. Thus, compound (23) was annotated as luteolin-O-hexoside53. Luteolin-7-O-glucoside was previously isolated from the aerial parts of I. sisyrinchium34.
Flavanones
Compound (32) presented a molecular ion peak [M-H]- at m/z 301. Its MS2 profile exhibited characteristic fragment ion peaks at m/z 286 [M-H-CH3]- due to loss of a methyl moiety, m/z 164 corresponds to [M-H-C7H5O3]- and m/z 151 produced by RDA cleavage fragmentation at 2, 3-position of C-ring in the Flavanone54. Besides typical fragments were observed at m/z 111, m/z 8755. Thus, compound (32) was identified as hesperetin. It was previously reported in the rhizomes of I. tectorum Maxim34.
Isoflavonoids.
They comprise a huge, distinguished class of secondary metabolites isolated from the genus Iris34. Compound (25) showed a deprotonated molecular ion [M-H]- at m/z 461. The MS2 profile exhibited characteristic fragment ion peaks at m/z 299 [M-H-162]- corresponding to the loss of O-hexosyl moiety, m/z 284 [M-H-162-CH3]- and a radical product ion at m/z 283 [M-H-162-CH3-H]-· with high relative abundance. Other distinctive fragment ion peaks at m/z 255 corresponded to a subsequent loss of CO molecule from m/z 283 while m/z 240 attributed to the loss of CO2 molecule from m/z 28456,57. Thus, compound (25) was tentatively identified as tectoridin. It was previously reported in the rhizomes I. spuria34.
Compound (28) displayed a molecular ion peak [M-H]- at m/z 315. Its MS2 profile showed the diagnostic base peak at m/z 300 owing to [M-H-CH3]- and two characteristic fragments at m/z 272 [M-H-CH3-CO]-and m/z 227 [M-H- CH3-CO-CO2]- due to a subsequent loss of CO, followed by CO2 29,58. Thus, compound (28) was tentatively identified as tetrahydroxy-methoxyisoflavone (irilin D). Compound (29) exhibited a pseudomolecular ion peak at m/z 299 [M-H]–. Its MS2 profile exhibited characteristic fragment ion peaks at m/z 284 (base peak) corresponding to [M-H-CH3]–, the radical aglycone ion at m/z 283 [Y˚-H]– and m/z 240 [M-H-CH3-CO2]- due to a subsequent loss of carbon dioxide. Thus, compound (29) was tentatively identified as trihydroxy-methoxyisoflavone (rhamnocitrin)59. Compound (30) showed a deprotonated molecular ion [M-H]- at m/z 269. Its MS2 profile exhibited characteristic fragment ion peaks at m/z 241 due to [M-H-CO]-, m/z 225 corresponding to [M-H-CO2] – followed by a subsequent loss of 2 CO molecules to yield m/z 169 as a fragment ion. Besides, fragment ions at m/z 201 attributed to [M-H-C3O2]- and m/z 133 due to [0,3B-] C-ring cleavage60. Thus, compound (30) was tentatively identified as genistein. Noteworthy, compound (28), (29), (30) which was previously reported in I. tectorum34.
Compound (31) displayed a molecular ion peak at m/z 301 in positive ionization mode and m/z 299 in negative ionization mode. The MS2 profile showed a characteristic fragment ion peak at m/z 286 due to [M+H-CH3]+ followed by a subsequent loss of H2O molecule to yield m/z 268 as a product ion. A fragment ion at m/z 183 was detected corresponding to [1,3A]+ RDA fragmentation arising from C-ring cleavage followed by a subsequent loss of CH3 to give the base peak at m/z 168. Aside, a product ion was detected at m/z 140 corresponding to [1,4A]+61,62. In the same context, inspecting fragmentation in the negative ion mode, the MS2 spectrum showed a diagnostic base peak at m/z 284 due to [M-H-CH3]-, m/z 212 corresponding to [284-CO2-CO]- and m/z 166 attributed to [0,3A]- arising from C-ring cleavage63. Thus, compound (31) was tentatively identified as trihydroxy-methoxyisoflavone (tectorigenin). Compound (34) showed a molecular ion peak at m/z 315 in positive ionization mode and m/z 313 in negative ionization mode. The MS2 spectrum showed a diagnostic base peak at m/z 300 is due to [M+H-CH3]+. A characteristic fragment ion peak at m/z 272 corresponding to [M+H-CO]+. The MS2 profile in negative ionization mode displayed a diagnostic base peak at m/z 298 owing to [M-H-CH3]- followed by a subsequent loss of another CH3 moiety to yield m/z 283. Besides characteristic fragment ion peaks at m/z 269 due to [M-H-CH3-CHO]-, m/z 252 attributed to [M-H-C2H5O2]-64,65. Thus, compound (34) was identified as dihydroxy-dimethoxyisoflavone (irisolidone). It was previously reported in the rhizomes I. germanica34.Compound (35) displayed a molecular ion peak at m/z 315 in the positive ionization mode and m/z 313 in the negative ionization mode. The MS2 profile in the positive ionization mode showed a characteristic fragment ion peak at m/z 300 due to [M+H-CH3]+ followed by a subsequent loss of H2O molecule to yield m/z 282 as a product ion. Other fragment ion peaks were detected at m/z 269 corresponding to [M+H-2*CH3-O]+, m/z 254 attributed to [M+H-CH3O2]+, m/z183 due to [M+H-C5H6O4]+ and m/z 168 corresponding to [1,3A]+ RDA fragmentation arising from C-ring cleavage. The MS2 spectrum in the negative ionization mode showed a diagnostic base peak at m/z 298 due to [M-H-CH3]- and a fragment ion at m/z 267 corresponding to [M-H-2CH3-O]-, m/z 255 attributed to [M-H-C3H6O] – and m/z 211 due to [M-H-C4H6O3]-55. Thus, compound (35) was identified as dihydroxy-dimethoxyisoflavone isomer (5,7-Dihydroxy-2',6-dimethoxyisoflavone). Compound (31) and (35) was previously isolated from I. pseudacorus rhizomes34.
Xanthone Derivatives
Iris species have been known as a rich source of xanthones66. Mangiferin, is the most abundant natural glycosylated xanthone and widely reported in literature. Compound (6) showed a molecular ion peak [M-H]– at m/z 583. Its MS2 profile exhibited characteristic fragment ion peaks at m/z 565 corresponding to [M-H-H2O]− , m/z 463 corresponding to [M-H-0,2X]− and m/z 331 [M-H-162-90]− due to 0,3X- fragmentation in C-linked hexose and loss of O-hexosyl moiety. The base peak at m/z 301 was assigned to [M−H−162−0,2X-]− and m/z 259 [M-H-(2 × 162)]- owing to the loss of two hexosyl units67. Thus, compound (6) was tentatively identified as neomangiferin. It was previously reported in I. dichotoma34. Compound (11) showed a molecular ion peak [M+H]+ at m/z 423. Its MS2 profile exhibited typical fragment ion peaks at m/z 351 attributed to [M+H-4H2O]+, m/z 339 [M+H-30-54]+ due to [2,3X+ -3*H2O], m/z 303, m/z 273 corresponding to [M+H-0,2X]+ , [M+H-0,1X]+, respectively and m/z 261 [M+H-162]+ due to loss of a hexosyl moiety68. Thus, compound (11) was tentatively identified as mangiferin. Besides in the positive ionization mode, the fragmentation pattern of mangiferin (11) showed a stronger relationship than with isomangiferin (13), because the base peak ion in mangiferin was noticed at m/z 273 (due to the location of C-2 glucose on the di benzo-γ-pyrone skeleton) but in isomangiferin the base peak ion was observed at m/z 303. In the negative ionization mode, compound (11) exhibited a deprotonated molecular ion [M-H]– at m/z 421. Its MS2 profile displayed characteristic fragment ion peaks at m/z 331, m/z 301, m/z 271 corresponding to 0,3X-,0,2X-, 0,1X- fragmentation in C-linked hexose respectively, and m/z 259 due to [M-H-162]-67. Mangiferin and isomangiferin were previously isolated from I. pseudacorus leaves34.
Fatty Acid Derivatives
Compound (36) eluted at Rt 16.43 min displayed a molecular ion peak [M-H]– at m/z 329. Its MS2 profile revealed characteristic fragment ion peaks at m/z 229 corresponding to the loss of the end-group HOCHCH(CH2)3CH3 from an oxylipin molecule. A product ion at m/z 211 was attributed to [M-H-C6H12O2]- and m/z 171 relative to [M-H-C9H14-H2O]-53. Thus, compound (36) was identified as trihydroxy-octadecenoic acid.
Identification of Other Compounds
Compound (37) eluted at Rt 21.18 min displayed a molecular ion peak [M+H]+ at m/z 261. Its MS2 spectrum displayed characteristic fragment ion peaks at m/z 243 due to [M+H-H2O]+, m/z 167 and m/z 93 corresponding to 1,3A+ and 1,3B+, respectively. A product ion at m/z 121 relative to [1,3A+- H2O-CO]+69. Thus, compound (37) was putatively identified as trihydroxy-methoxybenzophenone which was previously reported in the rhizomes of I. adriatica70 and I. pallida71.
In Vitro biological evaluation
In Vitro evaluation of antioxidant activity using DPPH assay
The antioxidant potential of I. pseudacorus extracts was evaluated using DPPH assay (Table 3). I. pseudacorus rhizomes extracts (IPR-J and IPR-E) exhibited the highest radical scavenging activity with % inhibition 75.84, 60.75 at a concentration of 125 μg/mL and IC50 values of 40.89 µg/mL, 97.97 µg/mL, respectively. Trolox was used as positive control exhibiting IC50 value at 14.59 µg/mL. While the lowest values were shown by aerial part methanol extracts of IPA-J and IPA-E with % inhibition 30.43 and 33.93 at concentration 125 μg/mL respectively.
Table 3.
DPPH, α-glucosidase, lipase and tyrosinase inhibitory activity of I. pseudacorus extracts.
| Sample | DPPH | α-glucosidase | Lipase | Tyrosinase | ||||
|---|---|---|---|---|---|---|---|---|
| %Inhibition | IC50 µg/mL | %Inhibition | IC50 µg/mL | %Inhibition | IC50 µg/mL | %Inhibition | IC50 µg/mL | |
| IPA-J | 30.43 | NC | NA | NC | 92.91 | 2.22±0.13 | NA | NC |
| IPR-J | 75.84 | 40.89± 0.92 | 99.96 | 18.52± 0.75 | 97.67 | 2.35± 0.03 | 9.43 | NC |
| IPA-E | 33.93 | NC | NA | NC | 95.99 | 0.42± 0.01 | NA | NC |
| IPR-E | 60.75 | 97.97± 3.44 | 67.70 | 57.89± 2.32 | 93.63 | 4.81± 0.09 | 10.34 | NC |
The results are calculated as mean ± SD, n=3.
NA, not active; NC, not calculated; IPA-J, Japanese I. pseudacorus aerial parts; IPR-J, Japanese I. pseudacorus rhizomes; IPA-E, Egyptian I. pseudacorus aerial parts; IPR-E, Egyptian I. pseudacorus rhizomes.
Enzyme inhibitory activity
In the current study, the enzyme inhibitory activity of I. pseudacorus extracts was assessed in vitro against tyrosinase, α-glucosidase and lipase enzymes. Results are displayed in Table 3.
In Vitro anti-hyperglycemic evaluation using α-glucosidase enzyme assay
The anti-hyperglycemic activity of I. pseudacorus extracts (IPA-J, IPR-J, IPA-E, and IPR-E) was evaluated in vitro using α-glucosidase inhibitory assay. Results revealed that I. pseudacorus rhizomes methanol extracts (IPR-J and IPR-E) exhibited the highest α-glucosidase inhibitory activity displaying percentage inhibition of 99.96, 67.70 at a concentration of 100 μg/mL and IC50 values are 18.52 µg/mL, 57.89 µg/mL, respectively being more potent as compared to acarbose with IC50 value of 362.088 µg/mL (Table 3). On the other hand, the aerial part methanol extracts of I. pseudacorus (IPA-J and IPA-E) showed no α-glucosidase inhibitory activity.
In Vitro anti- hyperlipidemic activity using pancreatic lipase enzyme assay
The antihyperlipidaemic activity of I. pseudacorus extracts (IPA-J, IPR-J, IPA-E, IPR-E) was evaluated in vitro for potential pancreatic lipase inhibitory activity. As shown in Table 3, all extracts of I. pseudacorus exerted significant lipase inhibitory activity and were potent compared to cetilistat used as positive control. The % inhibition of IPR-J, IPR-E, IPA-J, IPA-E was 97.67, 93.63, 92.91 and 95.99, respectively at a concentration of 25 μg/ml. Besides, IC50 values were 2.35 µg/mL, 4.81 µg/mL, 2.22 µg/mL and 0.42 µg/mL respectively compared to cetilistat with IC50 value of 7.47 µg/mL.
In Vitro anti-melanogenesis activity using tyrosinase enzyme assay
The tyrosinase inhibitory activity of the I. pseudacorus extracts (IPA-J, IPR-J, IPA-E and IPR-E) was evaluated in vitro. As shown in Table 3, no anti-melanogenesis activity was observed for all I. pseudacorus extracts up to 500 µg/mL. Arbutin was used as a positive control and displayed an IC50 value of 120 µg/mL.
Molecular docking studies
Molecular docking
Molecular docking studies were conducted for the major identified compounds in I. pseudacorus extracts within the active sites of human α-glucosidase (HAG) and human pancreatic lipase (HPL) (Table 4).
Table 4.
Free binding energies (∆G) of the major identified compounds in I. pseudacorus extracts within the active sites of human α-glucosidase (HAG) and human pancreatic lipase (HPL) using molecular docking and expressed in kcal/mol.
| Compound | C-docker energy ∆G (Kcal/mol) | |
|---|---|---|
| α-Glucosidase | Pancreatic lipase | |
| Quercetin (38) | − 44.02 | − 37.35 |
| 6-O-Galloylglucose (2) | − 43.09 | − 33.49 |
| Irilin D (28) | − 34.92 | − 31.51 |
| Rhamnocitrin (29) | − 30.66 | − 30.49 |
| Kaempferol-O-glucuronide (27) | − 30.11 | − 18.71 |
| Tectorigenin (31) | − 28.22 | − 25.65 |
| Genistein (30) | − 27.86 | − 20.63 |
| Mangiferin (11) | − 27.81 | − 22.55 |
| Luteolin-7-O‐ glucoside (23) | − 27.74 | − 19.90 |
| Apigenin-7-O-glucoside (40) | − 26.13 | − 13.62 |
| 5,7-Dihydroxy-2,6-Dimethoxyisoflavone (35) | − 25.18 | − 20.47 |
| Dihydrokaempferide (33) | − 24.94 | − 23.09 |
| Irisolidone (34) | − 24.85 | − 22.58 |
| Orientin (19) | − 24.49 | − 22.72 |
| Isovitexin (43) | − 23.11 | − 22.19 |
| Isomangiferin (13) | − 20.69 | − 15.67 |
| Vitexin (42) | − 19.54 | − 18.71 |
| tectoridin (25) | − 17.53 | − 10.40 |
| Isoorientin 6''-glucoside (10) | f.d. | − 6.96 |
| Schaftoside (18) | f.d. | − 5.82 |
| Vicenin-2 (22) | f.d. | − 7.01 |
| Neomangiferin (6) | f.d. | 6.34 |
| Isovitexin-7-O-glycoside (9) | f.d. | 9.42 |
|
Acarbose (3TOP co-crystallized inhibitor) |
− 59.61 | – |
| Cetilistat | – | − 51.24 |
| Methoxyundecylphosphinic acid (1LPB co-crystallized inhibitor) | – | − 30.34 |
f.d. failed to dock.
Positive values indicate unfavorable interaction.
Regarding human α-glucosidase, quercetin was the top hit compound, followed by galloyl glucose and then irilin D displaying free binding energy equals to − 44.02, − 43.09 and − 34.92 kcal.mol−1, respectively, approaching that of acarbose (the co-crystallized inhibitor) with ∆G=− 59.61 kcal.mol−1. Quercetin showed firm binding to HAG with formation of π − π hydrophobic interaction with Phe1560, Tyr1251 and Trp1355 as well as two conventional hydrogen bonds with Asp1157 and Asp1279 (Fig. 5A). Besides, galloyl glucose formed one π − π bond with Tyr1251, four conventional hydrogen bonds with Asp 1157, Lys1460 and Arg1510 and three C–H bonds with Asp1157 and Asp1526 (Fig. 5B). In the same context, irilin D was firmly bound to the catalytic residues of HAG by four π − π bonds with Phe1560, Tyr1251 and Trp1355; one conventional hydrogen bond with Asp1279 and one anion-π interaction with Asp1526 (Figure 5C). Noteworthy, acarbose displayed favorable interaction within the active sites of human α-glucosidase forming seven conventional hydrogen bonds with Asp 1526, Asp 1420, Asp1279, Lys1460 and Gln1158 and two C–H bonds with Asp1526 (Figure 5D).
Figure 5.
2D and 3D binding modes of quercetin (A), galloyl glucose (B), Irilin D (C), and acarbose (D) within the active sites of human α-glucosidase (HAG).
Similarly, molecular docking of the major metabolites identified in I. pseudacorus extracts within the active sites of human pancreatic lipase revealed favorable binding where quercetin, galloyl glucose and irilin D exhibited the best fitting scores with ∆G equals to − 37.35, − 33.49, − 31.51 kcal.mol−1 approaching that of cetilistat (an irreversible pancreatic lipase inhibitor) with ∆G = − 51.24 kcal.mol−1 and methoxyundecylphosphinic acid (MUP), the co-crystallized inhibitor and a reversible pancreatic lipase inhibitor, with ∆G = − 30.34 kcal.mol−1.
Quercetin showed strong π − π hydrophobic interaction with Phe215, Tyr114 and His263 in addition to three π-alkyl bonds with Leu264, Ala259 and Ala260 and two conventional hydrogen bonds with Arg256 and Ser152 (Fig. 6A). Meanwhile, galloyl glucose forms three conventional hydrogen bonds with Arg256 and Phe77; one π − π bond with Phe77 and three C–H bonds with Ser152, Gly76 and Phe77 (Fig. 6B). Besides, irilin D formed one conventional hydrogen bond with Arg256; four π-alkyl bond with Leu264, Ala259, Ala260, Ile78; one C–H bond with Phe215; one π-δ bond with Phe77 and four π − π bond with Tyr114, Phe 215 and Phe 77 (Fig. 6C). In the same context, cetilistat showed firm affinity to HPL with formation of five intermolecular hydrogen bonds with Gly76, Asp79, Ser152, Phe77 and His151 and one π − π bond with His263 (Fig. 6D). Furthermore, MUP formed two alkyl bonds with Arg 25 and Leu264; one π -alkyl bond with Pro180 and one π- donor hydrogen bond with Tyr114 (Fig. 6E). Noteworthy, Van der Waals forces represented a common interaction between all of these compounds and the amino acid residues present in the binding site of HPL and HAG enzymes.
Figure 6.
2D and 3D binding modes of quercetin (A), galloyl glucose (B), Irilin D (C), cetilistat (D), and methoxyundecylphosphinic acid MUP (1LPB co-crystallized inhibitor) (E) within the active sites of human pancreatic lipase (PL).
ADMET prediction
The purpose of ADMET prediction is to examine whether I. pseudacorus phytoconstituents possess drug-like properties or not. It is an essential step in the pharmaceutical R&D development. Descriptors of ADMET plot revealed that several compounds identified in I. pseudacorus extracts showed adequate intestinal absorption and aqueous solubility, thus, inferring good oral absorption. Moreover, they displayed low and undefined penetration through blood-brain barrier (BBB) and hence very low possibility for central nervous system (CNS) toxicity72. Besides, they were non-inhibitors for CYP2D6, thus, they could be easily excreted in phase 1 metabolism. Unfortunately, most of these constituents exhibited plasma protein binding PPB and hence fewer chances to reach to their targets (low bioavailability). Noteworthy, compounds (28, 29, 30, 31, 33, 34, 35 and 38) showed excellent intestinal absorption, as evidenced by their allocation in the 99% absorption ellipse (Fig. 7). Moreover, all compounds including cetilistat and acarbose exhibited very good solubility except compounds (6, 9, 10, 18 and 22). Besides, compounds (29, 30, 31, 33, 34 and 35) showed low penetration through BBB and hence were positioned inside the 99% BBB eclipse, concomitantly other compounds had undefined level of penetration and hence were positioned outside the 99% BBB eclipse (Fig. 7). Additionally, all compounds except (2, 18 and 22) displayed more than 90% PPB. However, compounds (2, 6, 9, 10, 11, 18, 22, 27, 34 and 35) including MUP were non-inhibitors for CYP2D6. Nevertheless, all compounds exhibited certain hepatotoxicity (Table 5)73.
Figure 7.
ADMET plot for bioactive metabolites identified in I. pseudacorus extracts displaying 95% and 99% confidence limit ellipses corresponding to blood-brain barrier (BBB) and human intestinal absorption models.
Table 5.
Absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties of major metabolites identified in Iris pseudacorus extracts.
| Compound name | BBB level | Absorption level | Solubility level | Hepato-toxicity | CYP2D6 | PPB level | AlogP98 | PSA 2D |
|---|---|---|---|---|---|---|---|---|
| 6-O-Galloylglucose (2) | 4 | 3 | 4 | 1 | 0 | 0 | 0 | 189.24 |
| Apigenin-7-O-glucoside (40) | 4 | 3 | 3 | 1 | 1 | 2 | 0 | 168.984 |
| Luteolin-7-O‐ glucoside (23) | 4 | 3 | 3 | 1 | 1 | 2 | 0 | 189.799 |
| Dihydrokaempferide(33) | 3 | 0 | 3 | 1 | 1 | 2 | 0 | 97.607 |
| Genistein (30) | 3 | 0 | 3 | 1 | 1 | 2 | 0 | 88.677 |
| Irilin D (28) | 4 | 0 | 3 | 1 | 1 | 2 | 0 | 118.422 |
| Irisolidone (34) | 3 | 0 | 3 | 1 | 0 | 2 | 0 | 85.722 |
| Isomangiferin (13) | 4 | 3 | 3 | 1 | 1 | 2 | 0 | 201.684 |
| Isoorientin 6''-Glucoside (10) | 4 | 3 | 2 | 1 | 0 | 2 | 0 | 281.991 |
| Isovitexin (43) | 4 | 3 | 3 | 1 | 1 | 2 | 0 | 180.869 |
| Isovitexin-7-O-glycoside (9) | 4 | 3 | 2 | 1 | 0 | 2 | 0 | 261.176 |
| Kaempferol 7-O-Glucuronide (27) | 4 | 3 | 3 | 1 | 0 | 2 | 0 | 207.1 |
| Mangiferin (11) | 4 | 3 | 3 | 1 | 0 | 2 | 0 | 201.684 |
| Neomangiferin (6) | 4 | 3 | 1 | 1 | 0 | 2 | 0 | 281.991 |
| Orientin (19) | 4 | 3 | 3 | 1 | 1 | 2 | 0 | 201.684 |
| Quercetin (38) | 4 | 1 | 3 | 1 | 1 | 2 | 0 | 130.308 |
| Rhamnocitrin (29) | 3 | 0 | 3 | 1 | 1 | 2 | 0 | 97.607 |
| Schaftoside (18) | 4 | 3 | 2 | 1 | 0 | 0 | 0 | 252.246 |
| Tectoridin (25) | 4 | 3 | 3 | 1 | 1 | 2 | 0 | 177.914 |
| Tectorigenin (31) | 3 | 0 | 3 | 1 | 1 | 2 | 0 | 97.607 |
| Vicenin-2 (22) | 4 | 3 | 2 | 1 | 0 | 0 | 0 | 273.061 |
| Vitexin (42) | 4 | 3 | 3 | 1 | 1 | 2 | 0 | 180.869 |
| 5,7-Dihydroxy-2',6-Dimethoxyisoflavone (35) | 3 | 0 | 3 | 1 | 0 | 2 | 0 | 85.722 |
| Cetilistat | 4 | 3 | 1 | 0 | 1 | 2 | 0 | 46.484 |
| Mup | 0 | 0 | 3 | 0 | 0 | 1 | 1 | 26.23 |
| Acarbose | 4 | 3 | 1 | 1 | 0 | 0 | 0 | 328.062 |
0, 1, 2, 3, and 4 denote very high, high, medium, low, and undefined penetration via BBB respectively. 0, 1, 2, and 3 signify good, moderate, poor, and very poor intestinal absorption, respectively. Aqueous solubility: 0, 1, 2, 3, 4, and 5 show extremely low, very low but possible, low, good, optimal, and too soluble, respectively. Hepatotoxicity: 0, non-toxic; 1, toxic. CYP2D6, cytochrome P450-14DM inhibition: 0, non-inhibitor; 1, inhibitor. PBB, plasma protein binding: 0, less than 90%; 1, more than 90%. AlogP98, atom-type partition coefficient (ALogP98). PSA 2D, 2D polar surface area in A2.
Discussion
Metabolic profiling of Egyptian and Japanese I. pseudacorus aerial parts and rhizomes crude extracts was carried out using UPLC-ESI-MS/MS analysis to obtain better insight into the phytochemical profile that may contribute to the studied biological activities. Forty-three compounds were tentatively identified herein. The identified metabolites belonged to various chemical classes including 33 flavonoids, 3 xanthones, 3 phenolic acid derivatives, an amino acid, a sugar, a benzophenone, and a fatty acid. Xanthones were detected only in the aerial parts of each cultivar.
The normal metabolic processes produce free radicals that possess unpaired electrons which are extremely reactive and result in cell injury74. In the current study the antioxidant potential of I. pseudacorus extracts was evaluated using DPPH assay. The mechanism of DPPH radical scavenging assay relies on electron transfer reaction, wherein DPPH acts as a radical3. The ability of I. pseudacorus extracts to scavenge free radicals could be correlated to the high polyphenolic content of this plant (mainly isoflavonoids and flavonoids), which are accumulated mainly in the rhizomes of the two cultivars and easily donate proton ions from their phenolic hydroxyl groups to scavenge free radicals into less reactive radicals. The tentatively identified constituents in IPR-J and IPR-E were previously reported to exert significant antioxidant activity75,76. The promising antioxidant activity of IPR-E could be attributed to tectoridin, tectorigenin, quercetin, irisolidone, genistein, rhamnocitrin, irilinD, dihydrokaempferide and 5,7-dihydroxy-2',6-dimethoxyisoflavone identified in the rhizome extract. In the same context vitexin, isovitexin and quercetin could be responsible for the observed activity of IPR-J75,76. The free radical scavenging abilities of tectorigenin (a metabolite formed by tectoridin metabolism via intestinal microflora) and found in IPR-E, were previously investigated in vivo. Tectorigenin scavenged intracellular free radicals and reduced lipid peroxidation in Chinese hamster lung protecting the viability of fibroblast (V79-4) cells exposed to hydrogen peroxide via the stimulation of extracellular signal regulated kinase (ERK)77. Besides, irisolidone exhibited promising antioxidant activity with IC50 value 12.62 μg/mL compared to propyl gallate (IC50 6.72 μg/mL)78. In addition, vitexin displayed promising free radical scavenging activity with IC50 value 31.4 μg/mL compared to trolox IC50 17.3 μg/mL79. Genistein was considered an effective antioxidant agent with IC50 value at 1.89 ± 0.16 μg/mL for DPPH compared to trolox IC50 value at 0.0247 ± 0.005 μg/mL80.
Several enzymes including α-glucosidase, lipase, and tyrosinase are considered as potential targets for lessening symptoms of diabetes mellitus, obesity and skin disorders, respectively1. Treatment of diabetes and its complications could be achieved by inhibiting key digestive enzymes as α-glucosidase that participate in starch digestion6. The anti-hyperglycemic activity of I. pseudacorus extracts (IPA-J, IPR-J, IPA-E, and IPR-E) was evaluated in vitro using α-glucosidase inhibitory assay. The promising anti-hyperglycemic activity of IPR-J and IPR-E could be attributed to richness in flavonoids, isoflavonoids and phenolic acids. These secondary metabolites were previously shown to possess anti-hyperglycemic activity via acting as potent α-glucosidase inhibitors75. Noteworthy, studies for α-glucosidase inhibitory activity of various Iris species are still scarce. The potent α-glucosidase inhibitory activity of IPR-E could be attributed to tachioside81, quercetin82,5,7-dihydroxy-2',6-dimethoxyisoflavone83,tectorigenin84 and genistein 85. In the same context, IPR-J activity could be attributed to vitexin6, isovitexin6, quercetin82 and apigenin-O-hexoside86. All these constituents act in synergistic mechanism with the other phenolic compounds found in IPR-E and IPR-J. Quercetin was recognized as an α-glucosidase inhibitor similar to acarbose. It was reported to delay glucose absorption and prevent the digestion of carbohydrates by inhibiting the sucrase, maltase and α-amylase. It also promotes proliferation of pancreatic beta cell, thus, improving absorption of glucose87. 5, 7-Dihydroxy-2', 6-dimethoxyisoflavone was reported as an effective anti-diabetic agent. It exhibited excellent activity against α-glucosidase enzyme with IC50 value at 0.321±0.008 µg/mL compared to acarbose IC50 value at 1.52±0.004 µg/mL at the same time it inhibited protein glycation strongly with % inhibition of 70.41 at concentration of 3 µg/mL as compared to rutin (82.50%). Noteworthy, inhibition of protein glycation lead to delay of diabetic complications as neuropathy and nephropathy83. An in vivo study on normoglycemic and induced diabetic rats examined the antihyperglycemic activity of vitexin and isovitexin. It was found that the highest reduction in postprandial blood glucose level was in induced diabetic rats treated orally with 200 mg/kg of vitexin and 100 mg/kg of isovitexin and the percentage of the reduction was similar to acarbose. Vitexin and isovitexin displayed strong in vitro α-glucosidase inhibition with IC50 values of 4.1 and 6.7 µg/mL compared with acarbose IC50 value at 4.3 × 10-2 µg/mL6. Genistein was reported as a potent α-glucosidase inhibitor, it remarkably inhibited α-glucosidase enzyme with IC50 values of 40.09 ± 0.94 µg/mL compared with acarbose IC50 value at 296.6 ± 1.06 µg/mL 85. To the best of our knowledge, the present study is the first regarding the anti-hyperglycemic activity of I. pseudacorus. At this point, the presented results could open novel perspectives for designing new plant-based nutraceuticals.
The inhibition of pancreatic lipase is considered a precious approach for the management of diet-induced hyperglycemia (one of causes of diabetes mellitus) and obesity. The effectiveness of I. pseudacorus extracts as a promising anti-hyperlipidemic could be attributed to its richness in flavonoids. These secondary metabolites were previously showed to possess anti-hyperlipidaemic activity via acting as potent pancreatic lipase inhibitors. Noteworthy, few studies evaluated the pancreatic lipase inhibitory activity of various Iris species. Several phytoconstituents identified herein in I. pseudacorus extracts were previously reported as natural pancreatic lipase inhibitory compounds including schaftoside 88, orientin 89, isovitexin 89, kaempferol-3-O-rutinoside90, quercetin91, genistein92. Pancreatic lipase enzyme displayed a binding pocket for quercetin. Once quercetin was attached with the lipase resulted in conformation changes lessening the substrate - enzyme affinity. Quercetin pre-administration in rats (5 and 10 mg/kg) through in vivo studies lead to a remarkable decrease in rat fat absorption and increase its excretion. Quercetin strongly inhibited pancreatic lipase enzyme through in vitro studies with IC50 value at 70 μg/mL compared with orlistat IC50 80 μg/mL91. Schaftoside exhibited a potent pancreatic lipase inhibitory activity with % inhibition of 95.5% at a concentration of 250 μg/mL and IC50 value of 130 μg/mL compared with orlistat IC50 value at 98.80 μg/mL 88. Kaempferol-3-O-rutinoside showed effective pancreatic lipase inhibitory activity with IC50 value of 1.7± 0.30 μg/mL compared to orlistat IC50 0.72 ± 0.07 μg/mL90.
In silico molecular docking studies were performed to further confirm the obtained in vitro results and identify the possible interaction mechanisms between I. pseudacorus phytoconstituents within the active sites of human α-glucosidase (HAG) and human pancreatic lipase (HPL). The firm fitting between the hit compounds and the enzyme active sites could be attributed to the formation of several bonds including π-π bond, H-bond, C-H bond and Van der Waals forces with the amino acid moieties in the enzyme binding site. Besides, ADMET prediction was conducted to examine whether I. pseudacorus phytoconstituents possess drug-like properties or not. It is an essential step in the pharmaceutical R&D development.
Materials and methods
Chemicals and reagents
2, 2-Diphenyl-1-picrylhydrazyl (DPPH), pancreatic lipase enzyme from porcine animal, tyrosinase enzyme from mushroom plant and α-glucosidase enzyme from Saccharomyces cerevisiae fungi were purchased from the Sigma Aldrich, Japan. Arbutin and aluminum chloride (III) were obtained from Nacalai Tesque, Japan. Cetilistat was purchased from Combi-Blocks, United States of America. Acarbose and Trolox were obtained from Wako Pure Chemical Industries, Japan. Methanol was purchased from Al-Nasr Pharmaceutical Company, Egypt.
Plant material
Aerial parts and rhizomes of I. pseudacorus were collected in January 2019 from El-Orman botanical garden, Giza, Egypt (30°01′45″ N 31°12′47″ E). The plant was identified and authenticated morphologically by Eng. Therease Labib, consultant of plant taxonomy at the Ministry of Agriculture, National Gene Bank and El-Orman Botanical Garden, Egypt. The collection complied with the IUCN Policy Statement on Research Involving Species at Risk of extinction and collection requirements were carefully followed in the conduct of this research to comply with institutional, national, and international guidelines and legislation. A voucher specimen (No. PHG-P-IP-417) was deposited in the Herbarium of Pharmacognosy Department, Faculty of Pharmacy, Ain Shams University. Aerial parts and rhizomes of I. pseudacorus cultivated in Japan were collected from the Medicinal Plant Garden of Kumamoto University, Japan (N32.794649, E130.72206), in December 2018, and were authenticated by Mr. Masato Watanabe, School of Pharmacy, Kumamoto University in Japan. A voucher specimen (No. 20181201-001) was deposited in the Herbarium of Medicinal Plant Garden of Kumamoto University. The metabolic profiling of aerial parts and rhizomes of Egyptian and Japanese I. pseudacorus extracts was carried out at the Center for Drug Discovery Research and Development, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt. Meanwhile, in vitro assays were performed at the school of pharmacy, Kumamoto University in Japan.
Preparation of plant extracts
The dried aerial parts and rhizomes of I. pseudacorus cultivars were air-dried in the shade, cut into small pieces. Egyptian and Japanese aerial parts of I. pseudacorus, 57 g and 11.1 g, respectively, besides, 69 g of Egyptian rhizomes and 10 g of Japanese rhizomes were extracted twice with 100% methanol (3L x 2) using a sonicator bath (690 HTAE Crest) for 60 minutes each time. Extracts were filtered by cotton fabric filter media. Extracts were concentrated using rotatory vacuum evaporator (Büchi, Switzerland) under reduced pressure at 45 °C and completely dried using a lyophilizer (Christ, Alpha 1–2 LD Plus) to yield 6 g, 1.16 g, 3 g and 0.43 g dried extracts of IPA-E, IPA-J, IPR-E and IPR-J, respectively.
Phytochemical analysis
UPLC-ESI-MS/MS characterization of I. pseudacorus extracts
Metabolic profiling of aerial parts and rhizomes of Egyptian and Japanese I. pseudacorus extracts was carried out on a Waters Xevo TQD mass spectrometer with UPLC Acquity mode (Milford, CT, USA) at the Center for Drug Discovery Research and Development, Faculty of Pharmacy, Ain Shams University. Extracts were liquefied in diluted methanol and injected directly into the UPLC-ESI-MS system. Both negative and positive ESI ionization modes were applied under the following conditions: A gradient of water and acetonitrile (ACN) with 0.1% was applied from 2 to 100% ACN in 60 min at 30 ◦C. The flow rate was 0.5 mL/min. The injection volume was 20 µL. The capillary voltage of MS (10 V), the ions were noticed within a mass range from 50 to 2000 m/z with collision energy (35 eV).
In Vitro biological evaluation
In Vitro evaluation of antioxidant activity using DPPH assay
The antioxidant activity of the yellow flag extracts was determined using DPPH scavenging assay in accordance with Shimamura et al. with minimal adjustments 93. The reaction mixture in 96 well culture plate contained. I. pseudacorus extracts (25 μL with several concentration ranges till 125 µg/mL), MES buffer (50 μL as volume and concentration =200 mM at pH = 6.0), 50% diluted ethanol (75 μL) mixed with 2, 2-diphenyl-1-picrylhydrazyl solution in EtOH (50 μL, 800 μM). After 20 min of incubation of the reaction mixture at 25 °C. The degree of bleaching of the violet tint of DPPH depends on the ability of I. pseudacorus extracts for hydrogen/electron donation and was measured spectrophotometrically at 520 nm of wavelength. Noteworthy, trolox (an artificial anti-oxidant agent) was used as a positive control. The percentage of antioxidant capacity of the yellow flag extracts equals to [] *100 Where Ac, As represents the control absorbance and the I. pseudacorus extract absorbance, respectively. The graph was plotted and the inhibitory concentration 50 (IC50) value was calculated93.
Enzyme inhibitory activity
In Vitro anti-hyperglycemic activity using α-glucosidase enzyme assay
The anti-hyperglycemic potential of the yellow flag extracts was conducted in accordance with Jabeen et al. method with slight alterations 93. The reaction mixture in 96 well culture plate contained a solution of α-glucosidase enzyme dissolved in phosphate buffer (10 μL, 1 UN/mL), 10 μL of the tested I. pseudacorus extract. Noteworthy, the range of extracts concentration for the α-glucosidase inhibition assay was 1.56 to 100 μg/mL mixed with phosphate buffer (60 μL, 0.2 M at pH 6.8). The mixture was incubated at 37 °C for 5 min. Subsequently, p-nitrophenyl α-D-glucoside (20 μL, 4 mM) was added to the reaction mixture as a substrate. Then, the reaction mixture was kept warm for 12 min at 37 °C. The amount of p-nitrophenol liberated by α-glucosidase enzyme was measured spectrophotometrically at 405 nm. The percentage of α-glucosidase inhibition potential of yellow flag extract was evaluated using the following equation: [1 – ]*100, in which Aa, Ab, Ac and Ad signify α-glucosidase and tested extracts absorbance, tested extracts absorbance only, α-glucosidase absorbance only, absorbance in absence of both, respectively. Acarbose (an α-glucosidase inhibitor) was used as a positive control. The graph was plotted and the concentration required to inhibit half-life of α-glucosidase function (IC50) was obtained 93.
In Vitro anti- hyperlipidaemic activity using pancreatic lipase enzyme assay
The anti-hyperlipidemic potential of yellow flag extracts was conducted by Bitou et al. method with minor alterations93. In an ELISA microplate reader, pancreatic lipase solution (50 μL at pH 7.4) in phosphate buffer (100 μg/mL, 0.2 M) was added together with 50 μL of I. pseudacorus extract at a concentration ranging from 1.56 to 100 μg/mL. After 10 min of incubation at 25 °C. 4-methylumbelliferyl oleate (4MUFO) (100 μL, 0.5 mM) was added to the reaction mixture as a substrate. The quantity of 4-methylumbelliferone liberated by the lipase enzyme was measured fluorometrically using a spectrophotometer at 355 nm excitation wavelength and 460 nm emission wavelength. Cetilistat (an artificial lipase inhibitor) was used as a positive control. The percentage of lipase inhibition was calculated using the following equation: [1- ]*100], where As and Ac symbolize I. pseudacorus extract and cetilistat absorbances. The graph was plotted and the concentration required to inhibit half-life of lipase activity (IC50) was obtained93.
In Vitro anti-melanogenesis activity using tyrosinase enzyme assay
The anti-melanogenesis potential of the yellow flag extracts was evaluated in accordance with Adhikari et al. method with minor changes93. The reaction mixture in 96 well culture plate contained phosphate buffer (120 μL at pH 6.8), tyrosinase solution in phosphate buffer (50 μL, 100 u/mL)) and 10 μL of I. pseudacorus extract. After 10 min of incubation at 25 °C, 20 μL of 2 mM of L-tyrosine substrate was added. The spectrophotometric absorbance was acquired at 476 nm after two and ten min. The percentage of tyrosinase inhibition was determined employing the following equation, [1- ]* 100, where As represents the absorbance difference of I. pseudacorus extract at the incubation time ten and two min and Ac denotes the absorbance difference of arbutin at the incubation time of ten and two min. Arbutin (a tyrosinase inhibitor) was used as a positive control. The graph was plotted and the concentration required to inhibit half-life of tyrosinase function (IC50) was obtained93.
Molecular docking studies
Molecular docking
In silico molecular docking study was performed on the major compounds identified in Iris pseudacorus extracts to elucidate the putative binding mode to the active sites of α-glucosidase and pancreatic lipase in an attempt to predict their probable mode of action as anti-hyperglycemic and anti-hyperlipedemic. Crystal structures of human α-glucosidase (HAG) (PDB ID 3TOP; 2.88 Å) and human pancreatic lipase (HPL) (PDB ID 1LPB; 2.46 Å) were retrieved from the protein data bank (www.pdb.org, accessed on 24 September 2022). Discovery Studio 4.5 (Accelrys Inc., San Diego, CA, USA) was employed applying the C-docker protocol as previously described 94.
ADMET prediction
Absorption, distribution, metabolism, excretion, and toxicity (ADMET) were predicted for the major metabolites identified in I. pseudacorus extracts employing ADMET prediction protocol using Discovery Studio 4.5 (Accelrys Inc., San Diego, CA, USA).
Statistical analysis
DPPH and enzyme inhibitory assays were carried out in triplicates, and the values were expressed as mean ± standard deviation. For the determination of the in vitro antioxidant, tyrosinase, lipase, and α-glucosidase inhibition potential, the (IC50) was estimated from the graph plots of the dose–response curves at each sample concentration by GraphPad Prism software (San Diego, CA, USA). The IC50 was defined as the concentration of the sample required to inhibit 50% of the tested enzyme activity.
Conclusions
The current study investigated for the first time the phytochemical diversity of I. pseudacorus aerial parts and rhizomes from Egypt and Japan. Besides, their potential inhibitory activity on selected enzymes (α-glucosidase, lipase and tyrosinase) were evaluated for the first time. Furthermore, their antioxidant capacities were assessed. Additionally, in Silico studies were performed to identify the possible interaction mechanisms between I. pseudacorus phytoconstituents and their targets to further validate the obtained in vitro results. Furthermore, ADMET prediction were conducted to evaluate their pharmacokinetics, pharmacodynamics and toxicity properties. Iris metabolites profiling was performed using UPLC-ESI-MS/MS analysis in an attempt to correlate the identified metabolites with the observed activities. Metabolites profiling revealed richness of I. pseudacorus extracts with biologically active compounds including schaftoside, orientin, isovitexin, tectorigenin, genistein, irilin D, quercetin and irisolidone. The rhizome methanol extracts of Egyptian and Japanese I. pseudacorus showed significant antioxidant activity and antihyperglycemic activity. Moreover, all investigated I. pseudacorus extracts showed potent anti-hyperlipidaemic activity via lipase enzyme inhibition. Results revealed that the rhizomes methanol extract of Japanese I. pseudacorus has the highest antioxidant, antihyperglycemic and anti-hyperlipidaemic activity among the other investigated extracts. Additionally, in silico molecular docking revealed that quercetin, galloyl glucose, and irilin D exhibited the highest fitting scores within the active sites of human α-glucosidase and pancreatic lipase. Moreover, most of phytoconstituents displayed promising pharmacokinetics, good pharmacodynamics and tolerable toxicity properties in ADMET plot. Consequently, the current study revealed that Egyptian and Japanese I. pseudacorus rhizomes may be considered as a promising natural source of antioxidant and antidiabetic agents. Furthermore, I. pseudacorus rhizomes and aerial parts of each cultivar should be taken into consideration as a valuable source for innovating new phytopharmaceuticals in the field of obesity as antihyperlipidaemic drugs. It is highly recommended to conduct further research for isolation of pure compounds from Egyptian and Japanese I. pseudacorus to explore the putative underlying mechanisms for the observed antihyperglycemic and antihyperlipidaemic activities. Additionally, further research should be conducted for elucidating in vivo enzyme inhibition activity besides, in depth studies are needed to confirm their efficacy and safety.
Author contributions
S.M.Y., M.W. and H.P.D. prepared the plant extracts, performed the experiments and data analysis. I.M.A. performed the molecular docking studies. S.M.Y. wrote the manuscript. I.M.A., H.P.D. and A.N.B.S. contributed to the study design, supervised the work, and reviewed the manuscript. All authors have approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors acknowledge the Science, Technology & Innovation Funding Authority (STDF) in cooperation with Egyptian Knowledge Bank (EKB) in Egypt for covering open access publishing fees.
Data availability
Data are available upon request from the first author, Suzan M. Yehia.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Iriny M. Ayoub, Email: irinyayoub@pharma.asu.edu.eg
Abdel Nasser B. Singab, Email: dean@pharma.asu.edu.eg
References
- 1.Mocan A, et al. Biological effects and chemical characterization of Iris schachtii Markgr. extracts: A new source of bioactive constituents. Food Chem. Toxicol. 2018;112:448–457. doi: 10.1016/j.fct.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayoub IM, et al. Anti-allergic, anti-inflammatory, and anti-hyperglycemic activity of Chasmanthe aethiopica leaf extract and its profiling using LC/MS and GLC/MS. Plants. 2021;10:1118. doi: 10.3390/plants10061118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzgaia N, Lee SY, Rukayadi Y, Abas F, Shaari K. Antioxidant activity, α-glucosidase inhibition and UHPLC–ESI–MS/MS profile of Shmar (Arbutus pavarii Pamp) Plants. 2021;10:1659. doi: 10.3390/plants10081659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos CM, Freitas M, Fernandes E. A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem. 2018;157:1460–1479. doi: 10.1016/j.ejmech.2018.07.073. [DOI] [PubMed] [Google Scholar]
- 6.Choo C, Sulong N, Man F, Wong T. Vitexin and isovitexin from the leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. J. Ethnopharmacol. 2012;142:776–781. doi: 10.1016/j.jep.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 7.Mane MP, Patil RS, Magdum AB, Narayankar CU, Nimbalkar MS. Evaluation of antioxidant and pancreatic lipase inhibitory potential of Polygala glaucoides L. and Polygala erioptera DC. Int. J. Bot. Stud. 2021;6:1259–1264. [Google Scholar]
- 8.Stefanucci A, et al. Discovery of arginine-containing tripeptides as a new class of pancreatic lipase inhibitors. Fut. Med. Chem. 2019;11:5–19. doi: 10.4155/fmc-2018-0216. [DOI] [PubMed] [Google Scholar]
- 9.Kandhari S, et al. Expert opinion on current trends in hyperpigmentation management: Indian perspective. Int. J. Res. 2022;8:142. [Google Scholar]
- 10.Divya G, et al. Renoprotective effect of tectorigenin glycosides isolated from Iris spuria L. (Zeal) against hyperoxaluria and hyperglycemia in NRK-49Fcells. Nat. Product Res. 2021;35:1029–1034. doi: 10.1080/14786419.2019.1613396. [DOI] [PubMed] [Google Scholar]
- 11.Ayoub IM, et al. Probing the antiallergic and anti-inflammatory activity of biflavonoids and dihydroflavonols from Dietes bicolor. J. Nat. Prod. 2018;81:243–253. doi: 10.1021/acs.jnatprod.7b00476. [DOI] [PubMed] [Google Scholar]
- 12.Ayoub IM, et al. Volatile constituents of Dietes bicolor (Iridaceae) and their antimicrobial activity. Zeitschrift für Naturforschung C. 2015;70:217–225. doi: 10.1515/znc-2015-0164. [DOI] [PubMed] [Google Scholar]
- 13.Ayoub I, El-Shazly M, Lu M-C, Singab A. Antimicrobial and cytotoxic activities of the crude extracts of Dietes bicolor leaves, flowers and rhizomes. South African J. Bot. 2014;95:97–101. doi: 10.1016/j.sajb.2014.08.012. [DOI] [Google Scholar]
- 14.Tikhomirova E, et al. Chemical composition and content of polysaccharides from the yellow iris (Iris pseudacorus L.) rhizomes. Pharmacognosy J. 2020;12:52. doi: 10.5530/pj.2020.12.143. [DOI] [Google Scholar]
- 15.Michalak A, et al. Iris pseudacorus as an easily accessible source of antibacterial and cytotoxic compounds. J. Pharm. Biomed. Anal. 2021;195:113863. doi: 10.1016/j.jpba.2020.113863. [DOI] [PubMed] [Google Scholar]
- 16.Okba MM, et al. UPLC-ESI-MS/MS profiling of the underground parts of common Iris species in relation to their anti-virulence activities against Staphylococcus aureus. J. Ethnopharmacol. 2022;282:114658. doi: 10.1016/j.jep.2021.114658. [DOI] [PubMed] [Google Scholar]
- 17.Rasool, S. U. A. I. S. R. a. S. o. P. a. G. D. o. I. S. Rasool, Shayaq Ul Abeer. Iris Species : Review and study of phytochemical and genetic diversity of Iris species. LAP LAMBERT Academic Publishing , London (2013).
- 18.Frederick, G. A Supplement to the Pharmacopoeia: Being a Treatise on Pharmacology in general, S. Gosnell Printer, Little Queen Street, London, 25 (1821).
- 19.Tarbeeva D, et al. Polyphenolic metabolites from Iris pseudacorus roots. Chem. Nat. Comp. 2015;51:451–455. doi: 10.1007/s10600-015-1313-9. [DOI] [Google Scholar]
- 20.Ayoub, I. M. et al. Insights into the neuroprotective effects of Salvia officinalis L. and Salvia microphylla Kunth in the memory impairment rat model. Food Funct. (2022). [DOI] [PubMed]
- 21.Elshamy, A. I. et al. UPLC-qTOF-MS Phytochemical profile and antiulcer potential of Cyperus conglomeratus Rottb. alcoholic extract. Molecules25, 4234 (2020). [DOI] [PMC free article] [PubMed]
- 22.Elkady, W., Ayoub, I., Abdel-Mottaleb, Y., ElShafie, M. F. & Wink, M. Euryops pectinatus L. flower extract inhibits P-glycoprotein and reverses multi-drug resistance in cancer cells: A mechanistic study. Molecules (Basel, Switzerland)25, 647, 10.3390/molecules25030647 (2020). [DOI] [PMC free article] [PubMed]
- 23.Korany DA, et al. Protective effects of Brownea grandiceps (Jacq.) against ϒ-radiation-induced enteritis in rats in relation to its secondary metabolome fingerprint. Biomed. Pharm. 2022 doi: 10.1016/j.biopha.2021.112603. [DOI] [PubMed] [Google Scholar]
- 24.Ayoub IM, et al. Chemical profile of Cyperus laevigatus and its protective effects against thioacetamide-induced hepatorenal toxicity in rats. Molecules. 2022;27:6470. doi: 10.3390/molecules27196470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Raey MA, El-Hagrassi AM, Osman AF, Darwish KM, Emam M. Acalypha wilkesiana flowers: Phenolic profiling, cytotoxic activity of their biosynthesized silver nanoparticles and molecular docking study for its constituents as Topoisomerase-I inhibitors. Biocatal. Agricult. Biotechnol. 2019;20:101243. doi: 10.1016/j.bcab.2019.101243. [DOI] [Google Scholar]
- 26.Dias MI, et al. Nutritional composition, antioxidant activity and phenolic compounds of wild Taraxacum sect. Ruderalia. Food Res. Int. 2014;56:266–271. doi: 10.1016/j.foodres.2014.01.003. [DOI] [Google Scholar]
- 27.Yuan J, et al. Qualitative analysis and componential differences of chemical constituents in Taxilli Herba from different hosts by UFLC-Triple TOF-MS/MS. Molecules. 2021;26:6373. doi: 10.3390/molecules26216373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellati F, Orlandini G, Pinetti D, Benvenuti S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J. Pharm. Biomed. Anal. 2011;55:934–948. doi: 10.1016/j.jpba.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Xie GY, et al. Phenolic metabolite profiles and antioxidants assay of three Iridaceae medicinal plants for traditional Chinese medicine "She-gan" by on-line HPLC-DAD coupled with chemiluminescence (CL) and ESI-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2014;98:40–51. doi: 10.1016/j.jpba.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Finger D, et al. Antifungal bioassay-guided fractionation of an oil extract of Propolis. J. Food Qual. 2013;36:291–301. doi: 10.1111/jfq.12039. [DOI] [Google Scholar]
- 31.Pan S, Jun-Li H, Gang W, Bo-Yang Y, Min-Jian Q. Analysis of flavonoids and phenolic acids in Iris tectorum by HPLC-DAD-ESI-MSn. Chin. J. Nat. Med. 2010;8:202–207. doi: 10.3724/SP.J.1009.2010.00202. [DOI] [Google Scholar]
- 32.Engels C, et al. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012;46:557–562. doi: 10.1016/j.foodres.2011.04.003. [DOI] [Google Scholar]
- 33.Blythe, E. K., Demirci, B., Goger, F. & Tabanca, N. Characterization of volatile and polar compounds of Jiaogulan tea [Gynostemma pentaphyllum (Thunb.) Makino] by hyphenated analytical techniques. Asian J. Chem.29, 1285-1290, 10.14233/ajchem.2017.20467 (2017).
- 34.Iwashina, T. & Mizuno, T. Flavonoids and xanthones from the genus Iris: Phytochemistry, relationships with flower colors and taxonomy, and activities and function. Nat. Product Commun.15, 1934578X20937151 (2020).
- 35.Snoussi, M. et al. Phytochemical analysis, antimicrobial and antioxidant activities of Allium roseum var. odoratissimum (Desf.) Coss extracts. Ind. Crops Prod.89, 533–542 (2016).
- 36.Xiong, J., Grace, M. H., Esposito, D., Wang, F. & Lila, M. A. Phytochemical characterization and anti-inflammatory properties of Acacia mearnsii leaves. Nat. Product Commun.11, 1934578X1601100524 (2016). [PubMed]
- 37.Dos Santos C, et al. Antioxidative, antiproliferative and antimicrobial activities of phenolic compounds from three Myrcia species. Molecules. 2018;23:986. doi: 10.3390/molecules23050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Y, Li H, Lin B, Wang G, Qin M. C-glycosylflavones from the leaves of Iris tectorum Maxim. Acta Pharmaceutica Sinica B. 2012;2:598–601. doi: 10.1016/j.apsb.2012.10.007. [DOI] [Google Scholar]
- 39.Ferreres F, Gil-Izquierdo A, Andrade PB, Valentão P, Tomás-Barberán F. Characterization of C-glycosyl flavones O-glycosylated by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2007;1161:214–223. doi: 10.1016/j.chroma.2007.05.103. [DOI] [PubMed] [Google Scholar]
- 40.Deng, X., Gao, G., Zheng, S. & Li, F. Qualitative and quantitative analysis of flavonoids in the leaves of Isatis indigatica Fort. by ultra-performance liquid chromatography with PDA and electrospray ionization tandem mass spectrometry detection. J. Pharm. Biomed. Anal.48, 562–567 (2008). [DOI] [PubMed]
- 41.Carvalho, A. R. et al. Urtica spp.: Phenolic composition, safety, antioxidant and anti-inflammatory activities. Food Res. Int.99, 485-494 (2017). [DOI] [PubMed]
- 42.Taamalli A, et al. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015;26:320–330. doi: 10.1002/pca.2566. [DOI] [PubMed] [Google Scholar]
- 43.Lv Z, Dong J, Zhang B. Rapid identification and detection of flavonoids compounds from bamboo leaves by LC-(ESI)-IT-TOF/MS. BioResources. 2012;7:1405–1418. doi: 10.15376/biores.7.2.1405-1418. [DOI] [Google Scholar]
- 44.Barros L, et al. Phenolic profiles of in vivo and in vitro grown Coriandrum sativum L. Food Chem. 2012;132:841–848. doi: 10.1016/j.foodchem.2011.11.048. [DOI] [Google Scholar]
- 45.Lee, S.-H. et al. Phenolic profiling and quantitative determination of common sage (Salvia plebeia R. Br.) by UPLC-DAD-QTOF/MS. Eur. Food Res. Technol.244, 1637-1646 (2018).
- 46.Yao, H. et al. Screening and quantitative analysis of antioxidants in the fruits of Livistona chinensis R. Br using HPLC-DAD–ESI/MS coupled with pre-column DPPH assay. Food Chem.135, 2802-2807 (2012). [DOI] [PubMed]
- 47.Bakr, R. O., Bishbishy, E. & Helmy, M. Profile of bioactive compounds of Capparis spinosa var. aegyptiaca growing in Egypt. Revista Brasileira de Farmacognosia26, 514-520 (2016).
- 48.Pereira CA, Yariwake JH, McCullagh M. Distinction of the C-glycosylflavone isomer pairs orientin/isoorientin and vitexin/isovitexin using HPLC-MS exact mass measurement and in-source CID. Phytochem. Anal. 2005;16:295–301. doi: 10.1002/pca.820. [DOI] [PubMed] [Google Scholar]
- 49.Liu S, et al. Characterization of compounds and potential neuraminidase inhibitors from the n-butanol extract of Compound Indigowoad Root Granule using ultrafiltration and liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012;59:96–101. doi: 10.1016/j.jpba.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Dueñas, M., Sánchez-Acevedo, T., Alcalde-Eon, C. & Escribano-Bailón, M. T. Effects of different industrial processes on the phenolic composition of white and brown teff (Eragrostis tef (Zucc.) Trotter). Food Chem.335, 127331 (2021). [DOI] [PubMed]
- 51.Ferreres, F. et al. Anti-inflammatory properties of the stem bark from the herbal drug Vitex peduncularis Wall. ex Schauer and characterization of its polyphenolic profile. Food Chem. Toxicol.106, 8-16 (2017). [DOI] [PubMed]
- 52.Qiao X, et al. Qualitative and quantitative analyses of flavonoids in Spirodela polyrrhiza by high-performance liquid chromatography coupled with mass spectrometry. Phytochem. Anal. 2011;22:475–483. doi: 10.1002/pca.1303. [DOI] [PubMed] [Google Scholar]
- 53.Llorent-Martínez EJ, Spínola V, Gouveia S, Castilho PC. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crops Prod. 2015;69:80–90. doi: 10.1016/j.indcrop.2015.02.014. [DOI] [Google Scholar]
- 54.Jiao Q, et al. Metabolism study of hesperetin and hesperidin in rats by UHPLC-LTQ-Orbitrap MS n. Xenobiotica. 2020;50:1311–1322. doi: 10.1080/00498254.2019.1567956. [DOI] [PubMed] [Google Scholar]
- 55.Bhat, G. HPLC-DAD-ESI-MS/MS Identification and Characterization of Major Constituents of Iris crocea, Iris germanica and Iris spuria Growing in Kashmir Himalayas, India. J. Anal. Bioanal. Tech.5, 83. 10.4172/2155-9872.1000223 (2014).
- 56.Chen Y, Song W, Peng ZH, Ge BY, Han FM. Identification of metabolites of tectoridin in-vivo and in-vitro by liquid chromatography-tandem mass spectrometry. J. Pharm. Pharmacol. 2008;60:709–716. doi: 10.1211/jpp.60.6.0005. [DOI] [PubMed] [Google Scholar]
- 57.Gao, B., Ma, Y., Zhang, L. t. & Ren, Q. Identification and characterization of the chemical components of Iris tectorum Maxim. and evaluation of their nitric oxide inhibitory activity. Rapid Commun. Mass Spectrom.35, e8959 (2021). [DOI] [PubMed]
- 58.Zhang Y-Y, Wang Q, Qi L-W, Qin X-Y, Qin M-J. Characterization and determination of the major constituents in Belamcandae Rhizoma by HPLC–DAD–ESI-MSn. J. Pharm. Biomed Anal. 2011;56:304–314. doi: 10.1016/j.jpba.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 59.Chen G, Li X, Saleri F, Guo M. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules. 2016;21:1275. doi: 10.3390/molecules21101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fengqin W, Jiang K, Zuguang L. Purification and identification of genistein in Ginkgo biloba leaf extract. Chin. J. Chromatogr. 2007;25:509–513. doi: 10.1016/S1872-2059(07)60019-4. [DOI] [PubMed] [Google Scholar]
- 61.Kukula-Koch W, et al. Major secondary metabolites of Iris spp. Phytochem. Rev. 2013;14:51–80. doi: 10.1007/s11101-013-9333-1. [DOI] [Google Scholar]
- 62.Li S, et al. Ionic-liquid-based ultrasound-assisted extraction of isoflavones from Belamcanda chinensis and subsequent screening and isolation of potential α-glucosidase inhibitors by ultrafiltration and semipreparative high-performance liquid chromatography. J. Separ. Sci. 2017;40:2565–2574. doi: 10.1002/jssc.201700258. [DOI] [PubMed] [Google Scholar]
- 63.Zhang WD, et al. Identification of the major metabolites of tectorigenin in rat bile by liquid chromatography combined with time-of-flight and ion trap tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008;22:2677–2684. doi: 10.1002/rcm.3660. [DOI] [PubMed] [Google Scholar]
- 64.Mykhailenko O, et al. Qualitative and quantitative analysis of Ukrainian Iris species: A fresh look on their antioxidant content and biological activities. Molecules. 2020;25:4588. doi: 10.3390/molecules25194588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang G, Gong T, Kano Y, Yuan D. Screening for in vitro metabolites of kakkalide and irisolidone in human and rat intestinal bacteria by ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. B. 2014;947:117–124. doi: 10.1016/j.jchromb.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 66.Singab ANB, et al. Shedding the light on Iridaceae: Ethnobotany, phytochemistry and biological activity. Ind. Crops Prod. 2016;92:308–335. doi: 10.1016/j.indcrop.2016.07.040. [DOI] [Google Scholar]
- 67.Beelders, T., De Beer, D., Stander, M. A. & Joubert, E. Comprehensive phenolic profiling of Cyclopia genistoides (L.) Vent. by LC-DAD-MS and-MS/MS reveals novel xanthone and benzophenone constituents. Molecules19, 11760–11790 (2014). [DOI] [PMC free article] [PubMed]
- 68.Sun Y, et al. Rapid Identification of Polyphenol C-Glycosides from Swertia franchetiana by HPLC—ESI-MS—MS. J. Chromatogr. Sci. 2009;47:190–196. doi: 10.1093/chromsci/47.3.190. [DOI] [PubMed] [Google Scholar]
- 69.Zhu J, Yi X, Zhang J, Chen S, Wu Y. Rapid screening of brain-penetrable antioxidants from natural products by blood-brain barrier specific permeability assay combined with DPPH recognition. J. Pharm. Biomed. Anal. 2018;151:42–48. doi: 10.1016/j.jpba.2017.12.055. [DOI] [PubMed] [Google Scholar]
- 70.Alperth F, et al. Metabolic profiling of rhizomes of native populations of the strictly endemic Croatian species Iris adriatica. Plant Biosyst. 2019;153:317–324. doi: 10.1080/11263504.2018.1478906. [DOI] [Google Scholar]
- 71.Roger, B., Jeannot, V., Fernandez, X., Cerantola, S. & Chahboun, J. Characterisation and quantification of flavonoids in Iris germanica L. and Iris pallida Lam. resinoids from Morocco. Phytochem. Anal.23, 450-455 (2012). [DOI] [PubMed]
- 72.Nguyen PTV, Huynh HA, Truong DV, Tran T-D, Vo C-VT. Exploring aurone derivatives as potential human pancreatic lipase inhibitors through molecular docking and molecular dynamics simulations. Molecules. 2020;25:4657. doi: 10.3390/molecules25204657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elhady SS, Youssef FS, Alahdal AM, Almasri DM, Ashour ML. Anti-hyperglycaemic evaluation of Buddleia indica leaves using in vitro, in vivo and in silico studies and its correlation with the major phytoconstituents. Plants. 2021;10:2351. doi: 10.3390/plants10112351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ibrahim SRM, Mohamed GA, Zayed MF, Ross SA. 8-Hydroxyirilone 5-methyl ether and 8-hydroxyirilone, new antioxidant and α-amylase inhibitors isoflavonoids from Iris germanica rhizomes. Bioorg. Chem. 2017;70:192–198. doi: 10.1016/j.bioorg.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 75.Khatib S, Faraloni C, Bouissane L. Exploring the use of iris species: Antioxidant properties, phytochemistry, medicinal and industrial applications. Antioxidants. 2022;11:526. doi: 10.3390/antiox11030526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amin, H. I. M. Et al. Phytochemistry and biological activities of iris species growing in iraqi kurdistan and phenolic constituents of the traditional plant Iris postii. Molecules26, 10.3390/molecules26020264 (2021). [DOI] [PMC free article] [PubMed]
- 77.Kang KA, et al. Cytoprotective effect of tectorigenin, a metabolite formed by transformation of tectoridin by intestinal microflora, on oxidative stress induced by hydrogen peroxide. Eur. J. Pharmacol. 2005;519:16–23. doi: 10.1016/j.ejphar.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 78.Ibrahim S, Al-Ahdal A, Khedr A, Mohamed G. Antioxidant α-amylase inhibitors flavonoids from Iris germanica rhizomes. Revista Brasileira de Farmacognosia. 2017;27:170–174. doi: 10.1016/j.bjp.2016.10.001. [DOI] [Google Scholar]
- 79.Erenler R, et al. Bioassay-guided isolation, identification of compounds from Origanum rotundifolium and investigation of their antiproliferative and antioxidant activities. Pharm. Biol. 2017;55:1646–1653. doi: 10.1080/13880209.2017.1310906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kładna A, Berczyński P, Kruk I, Piechowska T, Aboul-Enein HY. Studies on the antioxidant properties of some phytoestrogens. Luminescence. 2016;31:1201–1206. doi: 10.1002/bio.3091. [DOI] [PubMed] [Google Scholar]
- 81.Rasheed RB, Hussain S, Syed SK. Phytochemistry, nutritional and medicinal value of kiwi fruit. Postepy Biologii Komorki. 2021;48:147–165. [Google Scholar]
- 82.Lee D, Park JY, Lee S, Kang KS. In vitro studies to assess the α-glucosidase inhibitory activity and insulin secretion effect of isorhamnetin 3-o-glucoside and quercetin 3-o-glucoside isolated from Salicornia herbacea. Processes. 2021;9:483. doi: 10.3390/pr9030483. [DOI] [Google Scholar]
- 83.Mosihuzzman M, et al. Studies on alpha-glucosidase inhibition and anti-glycation potential of Iris loczyi and Iris unguicularis. Life Sci. 2013;92:187–192. doi: 10.1016/j.lfs.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 84.Al-Maharik N. Isolation of naturally occurring novel isoflavonoids: An update. Nat. Prod. Rep. 2019;36:1156–1195. doi: 10.1039/C8NP00069G. [DOI] [PubMed] [Google Scholar]
- 85.Ning Z-W, et al. Identification of α-glucosidase inhibitors from cyclocarya paliurus tea leaves using UF-UPLC-Q/TOF-MS/MS and molecular docking. Food Funct. 2019;10:1893–1902. doi: 10.1039/C8FO01845F. [DOI] [PubMed] [Google Scholar]
- 86.Jia Y, Ma Y, Cheng G, Zhang Y, Cai S. Comparative study of dietary flavonoids with different structures as α-glucosidase inhibitors and insulin sensitizers. J. Agric. Food Chem. 2019;67:10521–10533. doi: 10.1021/acs.jafc.9b04943. [DOI] [PubMed] [Google Scholar]
- 87.Laya A, Koubala BB, Negi PS. Antidiabetic (α-amylase and α-glucosidase) and anti-obesity (lipase) inhibitory activities of edible cassava (Manihot esculenta Crantz) as measured by in vitro gastrointestinal digestion: Effects of phenolics and harvested time. Int. J. Food Properties. 2022;25:492–508. doi: 10.1080/10942912.2022.2050256. [DOI] [Google Scholar]
- 88.Fernando W, et al. Isolation, identification and characterization of pancreatic lipase inhibitors from Trigonella foenum-graecum seeds. South African J. Bot. 2019;121:418–421. doi: 10.1016/j.sajb.2018.10.023. [DOI] [Google Scholar]
- 89.Guo H, et al. Screening of lipase inhibitors from bamboo leaves based on the magnetic ligand fishing combined with HPLC/MS. Microchem. J. 2020;153:104497. doi: 10.1016/j.microc.2019.104497. [DOI] [Google Scholar]
- 90.Habtemariam S. Antihyperlipidemic components of Cassia auriculata aerial parts: Identification through in vitro studies. Phytother. Res. 2013;27:152–155. doi: 10.1002/ptr.4711. [DOI] [PubMed] [Google Scholar]
- 91.Zhou J-F, et al. Quercetin is a promising pancreatic lipase inhibitor in reducing fat absorption in vivo. Food Biosci. 2021;43:101248. doi: 10.1016/j.fbio.2021.101248. [DOI] [Google Scholar]
- 92.Cardullo N, Muccilli V, Pulvirenti L, Tringali C. Natural isoflavones and semisynthetic derivatives as pancreatic lipase inhibitors. J. Nat. Prod. 2021;84:654–665. doi: 10.1021/acs.jnatprod.0c01387. [DOI] [PubMed] [Google Scholar]
- 93.Dirar AI, et al. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. South African J. Bot. 2019;120:261–267. doi: 10.1016/j.sajb.2018.07.003. [DOI] [Google Scholar]
- 94.Gad H, Al-Sayed E, Ayoub I. Phytochemical discrimination of Pinus species based on GC–MS and ATR-IR analyses and their impact on Helicobacter pylori. Phytochem. Anal. 2021;32:820–835. doi: 10.1002/pca.3028. [DOI] [PubMed] [Google Scholar]
- 95.Fruehwirth S, et al. LC–MS/MS method validation for the quantitation of 1-kestose in wheat flour. J. Food Compos. Anal. 2021;100:103930. doi: 10.1016/j.jfca.2021.103930. [DOI] [Google Scholar]
- 96.Li S, et al. Rapid identification and assignation of the active ingredients in fufang banbianlian injection using HPLC-DAD-ESI-IT-TOF-MS. J. Chromatogr. Sci. 2016;54:1225–1237. doi: 10.1093/chromsci/bmw055. [DOI] [PubMed] [Google Scholar]
- 97.Piraud, M. et al. ESI‐MS/MS analysis of underivatised amino acids: a new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun. Mass Spectrom.17, 1297-1311 (2003). [DOI] [PubMed]
- 98.Wen-Yuan L, et al. Characterization and simultaneous quantification of multiple constituents in Aurantii Fructus Immaturus extracts by HPLC-DAD-ESI-MS/MS. Chin. J. Nat. Med. 2012;10:456–463. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from the first author, Suzan M. Yehia.