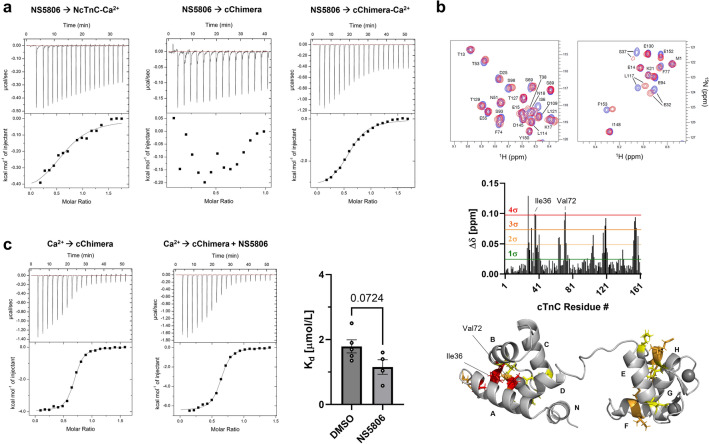
Figure 3.
Biophysical characterization of NS5806-cTnC interaction. (a) NS5806 binding to Ca2+-bound NcTnC (left), Ca2+-free cChimera (middle) and Ca2+-bound cChimera monitored by isothermal titration calorimetry (ITC). (b) Top: Part of 2D-1H-15N HSQC spectrum of cTnC in the absence (blue) and in the presence (purple) of NS5806 at roughly 1:1 stoichiometry. Middle: Plot of chemical shift perturbations (∆δ) of cTnC plotted against the amino acid sequence after addition of NS5806. Thresholds for different multiples of the standard deviation (SD) are shown in red. Bottom: Chemical shift perturbations mapped onto cTnC structure. Ile36 and Val72 are labelled accordingly. (c) ITC binding isotherm for Ca2+ titrated into cChimera in the absence (left) and in the presence (right) of NS5806. Means ± SEM, n = 4–5. Statistical significance between control and drug treatment was assessed with an unpaired two-tailed student’s t-test.