Abstract
The majority of neoplasms of the breast are derived from epithelial components and give rise to carcinoma, namely invasive ductal and lobular carcinoma of the breast. Unlike carcinomas, primary hematolymphoid malignancies of the breast are a rare group of malignant neoplasms. Due to their rarity, these patients’ epidemiological features and outcomes have not been studied well. A few limited case series and case reports suggest that this group of heterogeneous neoplasms has female predominance and poor prognosis. However, no systematic study exists to date. In order to bridge this knowledge gap, the National Cancer Institute’s Surveillance, Epidemiology, and End Results databases have been quarried and analyzed to investigate the epidemiological and outcome features of primary hematolymphoid malignancies of the breast. This study is one of the first efforts to establish a systematic understanding of the demographic characteristics and the survival features of this rare group of malignancies.
Keywords: Breast cancer, Lymphoma, Breast lymphoma, Primary lymphoma of breast, Non-Hodgkin lymphoma, Diffuse large B-cell lymphoma, Hodgkin lymphoma
Introduction
Primary hematolymphoid malignancies of the breast are among the rarest malignancies of the breast. The current WHO classification defines primary lymphoma of the breast as lymphomas confined to the breast or intramammary lymph nodes without any history of the previous diagnosis of lymphoma and any evidence of extramammary disease [1]. Given the dearth of lymphoid tissue within the breast, lymphoma arises in the mammary tissue on rare occasions. Initial studies suggested that less than 0.5% of all breast neoplasms fall under the hematolymphoid malignancy of the breast [2].
A few limited series have indicated that most primary breast lymphomas fall under the category of diffuse large B-cell lymphoma (DLBCL) [3–5]. Extranodal marginal zone lymphoma, follicular lymphoma, and aggressive Burkitt lymphoma have also been reported [3–10]. Amongst T cell lymphoma, peripheral T cell lymphoma, not otherwise specified (PTCL, NOS), has been reported. A unique subtype of anaplastic large T cell lymphoma (ALCL) that is negative for anaplastic lymphoma kinase (ALK) is known to occur specifically in the breast following some instances of breast implant and has been recognized as a separate entity now [11–15]. T cell lymphoma that usually arises in the skin and subcutaneous tissue such as subcutaneous panniculitis-like T-cell lymphoma (SPTL) has been reported in the breast arising from the overlying skin rather than deep mammary tissue [16–18]. The same can be said about another, more aggressive hematolymphoid malignancy, blastic plasmacytoid dendritic cell neoplasm (BPDCN), arising in the breast [19, 20].
Despite these few case reports and limited case series, there lacks a systematic study of this group of rare neoplasms. In order to bridge this knowledge gap, the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) databases have been quarried and analyzed to investigate the epidemiological and outcome features of primary hematolymphoid malignancies of the breast.
Materials and Methods
SEER Database Search
The National Cancer Institute’s SEER database is one of the world’s largest cancer databases, accounting for approximately 30% of the U.S. population. The program database was quarried for all histologically confirmed hematolymphoid cases with the breast designated as the primary site. A search was performed through the database to find hematolymphoid malignancies of the breast between 1975 and 2017. Only cases with the primary site designated as the breast were included (primary site coded as C50.1 – C50.9).
Statistical Analysis
Descriptive statistics were used for summarizing demographic and clinical variables. We used SPSS v.25 (IBM, NY, USA) to draw Kaplan–Meier curve to analyze the disease-specific survival associated with the age of diagnosis, tumor size, and each treatment modality. Log-rank (Mantel-Cox) test (pooled over strata) was also performed to analyze the effect of age of diagnosis, tumor size, and treatment modality in disease-free survival. A p-value of less than 0.05 was considered statistically significant.
Results
Patient Characteristics
1437 cases were found with primary hematological malignancies of the breast that have been diagnosed based on histopathological evaluation and fulfill our inclusion criteria. 1419 cases could be grouped as non-Hodgkin B cell lymphoma, T cell lymphoma, and Hodgkin lymphoma. The rest of the eighteen cases were of other hematolymphoid malignancies:
Eight patients had plasma cell neoplasm.
Three patients had BPDCN.
Six patients had myeloid sarcoma.
One patient was diagnosed with follicular dendritic cell sarcoma.
Next, the epidemiological features and outcomes of non-Hodgkin B cell lymphoma, T cell lymphoma, and Hodgkin lymphoma of the breast are analyzed.
Primary non-Hodgkin B cell Lymphoma of the Breast
A total of 1271 patients were diagnosed with non-Hodgkin B cell lymphoma making it the largest subset of hematolymphoid malignancy arising as primary breast hematolymphoid neoplasm. Most patients were diagnosed after the age of 50 showing a unimodal distribution peaking between the ages of 70 to 80 (Fig. 1 A). Most patients were female, with the female: male ratio of 24:1 (Fig. 1B). Most patients were Caucasian (83%) (Fig. 1 C). Of all different types of non-Hodgkin B cell lymphoma, DLBCL was the most commonly occurring breast lymphoma (Fig. 1D). The analysis of overall survival shows Burkitt lymphoma showing the worst outcome and follicular lymphoma showing the best outcomes of all different subtypes (Fig. 1E). The difference in overall survival between different subsets of non-Hodgkin B cell lymphoma patients was statistically significant (p = 0.03).
Fig. 1.
Demographic characteristics and outcome of patients with primary breast B cell lymphoma, non-Hodgkin type. (A) Bar graph showing age of diagnosis of primary breast non-Hodgkin B cell lymphoma patients. (B,C) Pie charts showing (B) sex and (C) racial distribution of primary breast non-Hodgkin B cell lymphoma patients. (D) Bar graph showing the distribution of subtypes of non-Hodgkin B cell lymphoma diagnosed as primary breast lymphoma (E) Kaplan-Meier curve showing comparison between overall survival of different primary breast non-Hodgkin B cell lymphoma patients
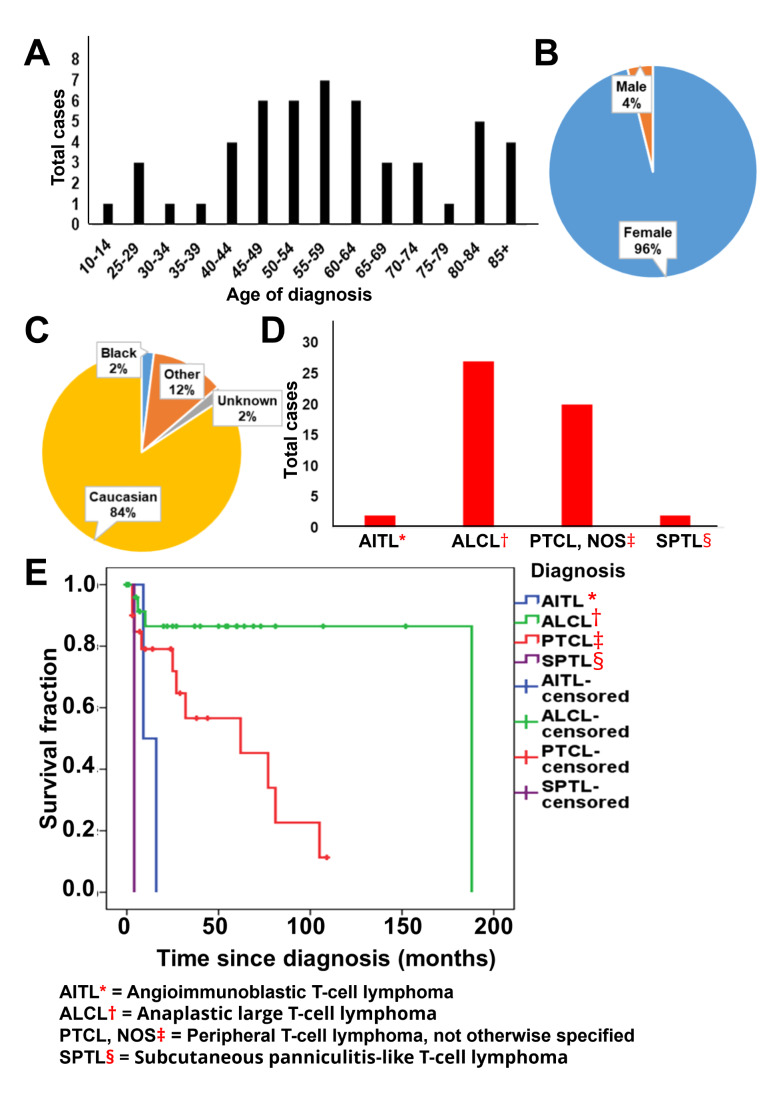
Primary T Cell Lymphoma of the Breast
Fifty-one patients were diagnosed with primary T cell lymphoma of the breast. The majority of the patients were diagnosed between the ages of 40 and 70 (Fig. 2 A). Similar to non-Hodgkin B cell lymphoma, most patients were female, with the female: male ratio of 24:1 (Fig. 2B). The majority of patients were Caucasian (84%) (Fig. 2 C). Amongst the T cell lymphoma, the most common diagnosis was ALCL (Fig. 2D). All ALCL in the database has been coded as implant associated ALCL. In terms of outcome, SPTL and AITL showed statistically significant poorer overall survival (p = 0.002).
Fig. 2.
Demographic characteristics and outcome of patients with primary breast T cell lymphoma. (A) Bar graph showing age of diagnosis of primary breast T cell lymphoma patients. (B,C) Pie charts showing (B) sex and (C) racial distribution of primary breast T cell lymphoma patients. (D) Bar graph showing the distribution of subtypes of T cell lymphoma diagnosed as primary breast lymphoma (E) Kaplan-Meier curve showing comparison between overall survival of different primary breast T cell lymphoma patients
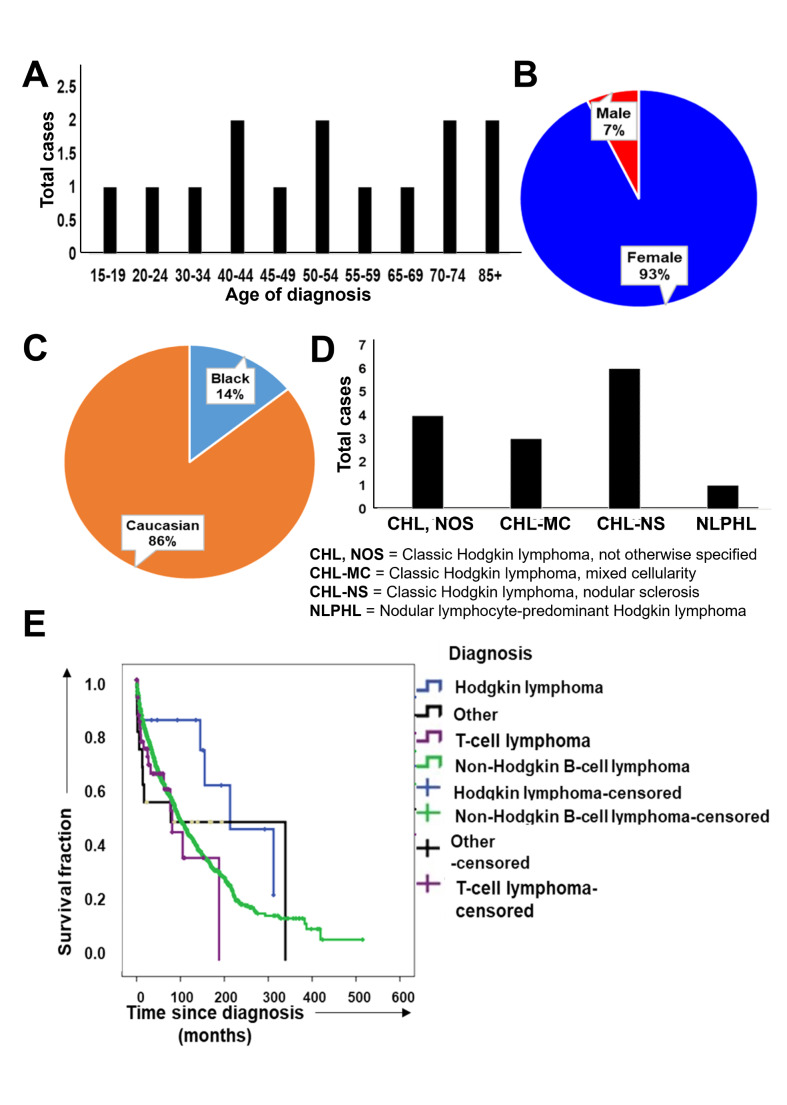
Primary Hodgkin Lymphoma of the Breast
Only seventeen cases of primary Hodgkin lymphoma of the breast were diagnosed. The distribution of age of diagnosis was scattered through all age groups, possibly due to a low number of cases (Fig. 3 A). The majority of patients were female (Fig. 3B) and Caucasian (Fig. 3 C). Most cases were diagnosed as classic Hodgkin lymphoma, nodular sclerosis subtype (Fig. 3D). Compared to primary breast T cell lymphoma, non-Hodgkin B cell lymphoma, and other types of hematolymphoid malignancies, the Hodgkin lymphoma patients performed well in terms of overall survival (p = 0.04) (Fig. 3E).
Fig. 3.
Demographic characteristics and outcome of patients with primary breast B cell lymphoma, Hodgkin type. (A) Bar graph showing age of diagnosis of primary breast Hodgkin lymphoma patients. (B,C) Pie charts showing (B) sex and (C) racial distribution of primary breast Hodgkin lymphoma patients. (D) Bar graph showing the distribution of subtypes of Hodgkin lymphoma diagnosed as primary breast lymphoma (E) Kaplan-Meier curve showing comparison between overall survival of different types of primary hematolymphoid malignancies of breast
Due to the low incidence of the disease, we used the longest period of time available in the database, even though some variables do not remain consistent over time. We wanted to understand how the outcome compares between patients diagnosed in a different era. We subdivided all patients, non-Hodgkin B cell lymphoma patients, and T cell lymphoma patients into four equal 11-year intervals (1975–85, 1986–96, 1997–2007, 2008–2018). Our analysis shows that primary breast non-Hodgkin B cell lymphoma’s outcome significantly improved with time (p = 0.035) (Fig. 4B). T cell lymphomas did not show a similar trend, possibly due to the mixing of all different subtypes that have different outcomes on their own (p = 0.78) (Fig. 4 C). The number of cases was not high enough for us to perform a meaningful analysis by separating all separate subtypes of T-cell lymphoma. No clear trend was seen with all lymphomas analyzed together (p = 0.1) (Fig. 4 A).
Fig. 4.
Outcome of patients with primary breast lymphoma diagnosed in different era. All patients were subdivided in equal 11-year intervals according to the year at diagnosis (1975–85, 1986–96, 1997–2007, 2008–2018). (A) Kaplan-Meier curve showing comparison between overall survival of patients with primary breast hematolymphoid neoplasms of all subtypes diagnosed in different era. (B) Kaplan-Meier curve showing comparison between overall survival of patients with primary breast non-Hodgkin B cell lymphomas of all subtypes diagnosed in different era. (C) Kaplan-Meier curve showing comparison between overall survival of patients with primary breast T cell lymphomas of all subtypes diagnosed in different era
Discussions
Hematolymphoid malignancies are one of the rarest groups of primary neoplasms of the breast. One of the significant problems that limit any study of this neoplasm is the lack of consensus on the definition of primary lymphoma of the breast. While some authors have characterized any lymphoma that presented with a predominant breast mass at the time of diagnosis as primary breast lymphoma, others have used more rigorous characterization and have excluded any lymphoma that had extra-mammary involvement. Some studies were limited by the lack of bone marrow biopsies to exclude systematic disease and only relied on patients’ symptoms and radiological features [21–24]. The current study is limited by its retrospective nature. Even though it is impossible to say how the cases were coded and if the primary diagnosis of breast lymphoma was made using a strict or a lenient criterion, there is no reason to believe that the coding will be biased towards one direction or the other. And hence, the total bias will likely be null.
Another limitation of the study is the inability to assess the effect of the stage on the patient outcome. Due to the long period used in the study, the stage reported is either absent, limited, or follows different iterations of the AJCC manual. The lack of data also does not allow us to standardize all stages according to the current AJCC manual. Ann arbor staging data, which would be more relevant for lymphoma, is also missing for more than 90% of the cases. Due to this inconsistency, we felt it is best to leave the stage of neoplasm out of the analysis. The effect of treatment on the outcome of these neoplasms is also an important factor to investigate. Unfortunately, the only treatment modality consistently available in the SEER database is the use of surgery at the primary site (or lack thereof). Since almost no patient received surgical resection as it is not the standard of care, and the chemo-radiation data was unavailable, we did not include the treatment modality in our analysis.
We observed better outcome for primary breast non-Hodgkin B cell lymphoma with more recent diagnosis. Although it is most likely that advancement of treatment modalities has driven this; it is also possible that the availability of better diagnostic tools has pushed for a stage migration (also known as the Will Roger phenomenon) which has led to this observation. With the limited data in our disposal, this is another question that remained unsolved.
In one of the most extensive single-center studies, Validire and colleagues investigated the factors affecting the primary DLBCL of the breast outcome. Breast involvement in a disseminated disease did not affect the overall or disease-free survival. DLBCL that started as breast primary but remained localized did not significantly show better outcomes than their disseminated counterparts [5]. Martinelli and colleagues studied the outcome of primary follicular lymphoma and extra-nodal marginal zone lymphoma of the breast and found that both tumors have an excellent prognosis, with more than 90% of patients reaching complete remission [10]. The prognosis of marginal zone lymphoma was better than follicular lymphoma; however, the distinction was not statistically significant [10].
The present study shows that most primary breast lymphomas are DLBCL and marginal zone lymphoma, respectively, confirming what earlier case series and case reports have indicated. Moreover, it shows a low incidence of primary breast Hodgkin lymphoma. Unlike DLBCL, Hodgkin lymphoma is predominantly a nodal disease. Given the limited number of lymph nodes in the mammary tissue, a low incidence of Hodgkin lymphoma is expected. Although the transplant-associated anaplastic large T cell lymphoma in the breast has been recently described, the overall incidence of all T cell lymphomas is low in the breast, owing to the low incidence of T cell lymphoma in general. T cell lymphoma is known for its aggressive course and poor outcome. This study shows that it remains true in the breast as well.
We have attempted to understand primary breast lymphoma using population-based data. We hope that our study will shed more light on this rare neoplasm and help future investigators better understand the knowledge gaps and questions that remain to be addressed about this uncommon but lethal malignancy.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Board WHOCoTE (2019) WHO Classification of Breast Tumours: WHO Classification of Tumours, vol 2. World Health Organization
- 2.Wiseman C, Liao KT. Primary lymphoma of the breast. Cancer. 1972;29:1705–1712. doi: 10.1002/1097-0142(197206)29:6<1705::AID-CNCR2820290640>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Ganjoo K, Advani R, Mariappan MR, McMillan A, Horning S. Non-Hodgkin lymphoma of the breast. Cancer. 2007;110:25–30. doi: 10.1002/cncr.22753. [DOI] [PubMed] [Google Scholar]
- 4.Talwalkar SS, Miranda RN, Valbuena JR, Routbort MJ, Martin AW, Medeiros LJ. Lymphomas involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol. 2008;32:1299–1309. doi: 10.1097/PAS.0b013e318165eb50. [DOI] [PubMed] [Google Scholar]
- 5.Validire P, Capovilla M, Asselain B, Kirova Y, Goudefroye R, Plancher C, Fourquet A, Zanni M, Gaulard P, Vincent-Salomon A, Decaudin D. Primary breast non-Hodgkin’s lymphoma: a large single center study of initial characteristics, natural history, and prognostic factors. Am J Hematol. 2009;84:133–139. doi: 10.1002/ajh.21353. [DOI] [PubMed] [Google Scholar]
- 6.Hugh JC, Jackson FI, Hanson J, Poppema S. Primary breast lymphoma. An immunohistologic study of 20 new cases. Cancer. 1990;66:2602–2611. doi: 10.1002/1097-0142(19901215)66:12<2602::AID-CNCR2820661224>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Giardini R, Piccolo C, Rilke F. Primary non-Hodgkin’s lymphomas of the female breast. Cancer. 1992;69:725–735. doi: 10.1002/1097-0142(19920201)69:3<725::AID-CNCR2820690320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Mattia AR, Ferry JA, Harris NL. Breast lymphoma. A B-cell spectrum including the low grade B-cell lymphoma of mucosa associated lymphoid tissue. Am J Surg Pathol. 1993;17:574–587. doi: 10.1097/00000478-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Ribrag V, Bibeau F, El Weshi A, Frayfer J, Fadel C, Cebotaru C, Laribi K, Fenaux P. Primary breast lymphoma: a report of 20 cases. Br J Haematol. 2001;115:253–256. doi: 10.1046/j.1365-2141.2001.03047.x. [DOI] [PubMed] [Google Scholar]
- 10.Martinelli G, Ryan G, Seymour JF, Nassi L, Steffanoni S, Alietti A, Calabrese L, Pruneri G, Santoro L, Kuper-Hommel M, Tsang R, Zinzani PL, Taghian A, Zucca E, Cavalli F. Primary follicular and marginal-zone lymphoma of the breast: clinical features, prognostic factors and outcome: a study by the International Extranodal Lymphoma Study Group. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2009;20:1993–1999. doi: 10.1093/annonc/mdp238. [DOI] [PubMed] [Google Scholar]
- 11.Keech JA., Jr Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554–555. doi: 10.1097/00006534-199708000-00065. [DOI] [PubMed] [Google Scholar]
- 12.Miranda RN, Medeiros LJ, Ferrufino-Schmidt MC, Keech JA Jr, Brody GS, de Jong D, Dogan A, Clemens MW (2019) : Pioneers of Breast Implant–Associated Anaplastic Large Cell Lymphoma: History from Case Report to Global Recognition. Plastic and reconstructive surgery. 143:7S-14S. 10.1097/PRS.0000000000005564 [DOI] [PubMed]
- 13.Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, De Jong D, Fayad LE, Amin MB, Haideri N, Bhagat G, Brooks GS. Breast implant–associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32:114. doi: 10.1200/JCO.2013.52.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roden AC, Macon WR, Keeney GL, Myers JL, Feldman AL, Dogan A. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: an indolent T-cell lymphoproliferative disorder. Mod Pathol. 2008;21:455–463. doi: 10.1038/modpathol.3801024. [DOI] [PubMed] [Google Scholar]
- 15.Wong AK, Lopategui J, Clancy S, Kulber D, Bose S. Anaplastic large cell lymphoma associated with a breast implant capsule: a case report and review of the literature. Am J Surg Pathol. 2008;32:1265–1268. doi: 10.1097/PAS.0b013e318162bcc1. [DOI] [PubMed] [Google Scholar]
- 16.Gualco G, Chioato L, Harrington WJ Jr, Weiss LM, Bacchi CE (2009) Primary and secondary T-cell lymphomas of the breast: clinico-pathologic features of 11 cases. Applied immunohistochemistry & molecular morphology: AIMM / official publication of the Society for Applied Immunohistochemistry. 17:301–306. 10.1097/PAI.0b013e318195286d [DOI] [PMC free article] [PubMed]
- 17.Schramm N, Pfluger T, Reiser MF, Berger F. Subcutaneous panniculitis-like T-cell lymphoma with breast involvement: functional and morphological imaging findings. Br J Radiol. 2010;83:e90–94. doi: 10.1259/bjr/69172676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong SI, Lim HS, Choi YR, Kim JW, Park MH, Cho JS, Lee JS, Kang HK. Subcutaneous panniculitis-like T-cell lymphoma of the breast. Korean J Radiol. 2013;14:391–394. doi: 10.3348/kjr.2013.14.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen KC, Su TC, Chen DR, Liou JH. A case report: Blastic plasmacytoid dendritic cell neoplasm is misdiagnosed as breast infiltrating ductal carcinoma. Int J Surg Pathol. 2015;23:84–88. doi: 10.1177/1066896914553662. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, Park HM, Ki SY, Choi YD, Yun SJ, Lim HS. Blastic plasmacytoid dendritic cell neoplasm of the breast: A case report and review of the literature. Med (Baltim) 2021;100:e25699. doi: 10.1097/md.0000000000025699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberman L, Giess CS, Dershaw DD, Louie DC, Deutch BM. Non-Hodgkin lymphoma of the breast: imaging characteristics and correlation with histopathologic findings. Radiology. 1994;192:157–160. doi: 10.1148/radiology.192.1.8208929. [DOI] [PubMed] [Google Scholar]
- 22.Yang WT, Lane DL, Le-Petross HT, Abruzzo LV, Macapinlac HA. Breast lymphoma: imaging findings of 32 tumors in 27 patients. Radiology. 2007;245:692–702. doi: 10.1148/radiol.2452061726. [DOI] [PubMed] [Google Scholar]
- 23.Jeanneret-Sozzi W, Taghian A, Epelbaum R, Poortmans P, Zwahlen D, Amsler B, Villette S, Belkacémi Y, Nguyen T, Scalliet P, Maingon P, Gutiérrez C, Gastelblum P, Krengli M, Raad RA, Ozsahin M, Mirimanoff RO. Primary breast lymphoma: patient profile, outcome and prognostic factors. A multicentre Rare Cancer Network study. BMC Cancer. 2008;8:86. doi: 10.1186/1471-2407-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surov A, Holzhausen HJ, Wienke A, Schmidt J, Thomssen C, Arnold D, Ruschke K, Spielmann RP. Primary and secondary breast lymphoma: prevalence, clinical signs and radiological features. Br J Radiol. 2012;85:e195–205. doi: 10.1259/bjr/78413721. [DOI] [PMC free article] [PubMed] [Google Scholar]