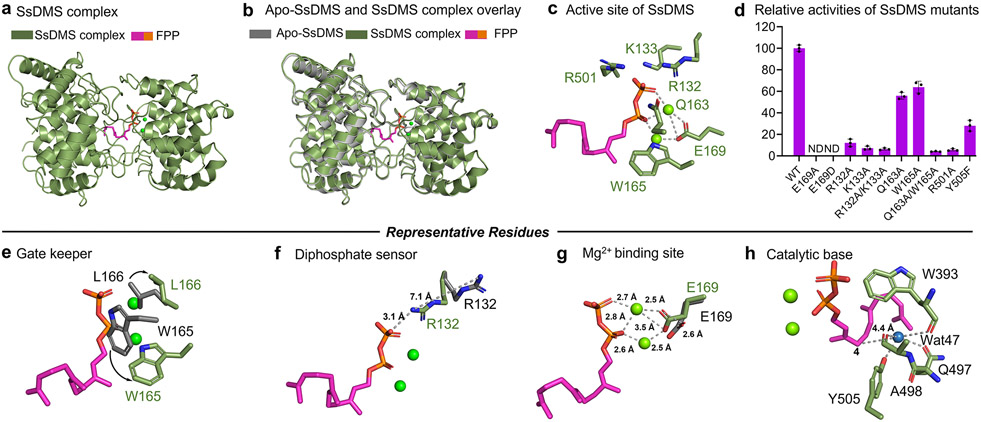
Figure 4. Structural snapshots of SsDMS in complex with ligand and divalent metal cofactor.
(a) SsDMS complex shows that FPP resides in the cleft of β and γ domain. (b) Overlaying apo-SsDMS and SsDMS complex shows minor conformational changes. (c) Active site of SsDMS. (d) Relative activities of SsDMS mutants reveal key residues in terpene cyclization. (e) W165, termed as a gatekeeper, rotates 90° and drives L166 outwards to allow the ligand access to enter the active site. (f) R132, termed as a diphosphate sensor, rotates its side chain to bring the guanidinium moiety within 3.1 Å of the diphosphate. (g) The E169 shifted by 2.6 Å to form a substrate-ions-enzyme coordinated complex. (h) The proposed catalytic base group deprotonates the final carbocation intermediate. The side chains in apo-SsDMS and SsDMS complex are shown in gray sticks and marine green sticks, respectively. Substrate of FPP is shown in magenta, the diphosphate group of which is shown in orange. ND, not detected. Error bars indicate the standard deviation of three independent replicates.