Abstract
The safety profile of COVID-19 vaccines is understudied in patients with systemic sclerosis (SSc). We compared short-term adverse events (AEs) 7 days following vaccination in patients with SSc vs other rheumatic (AIRDs), non-rheumatic autoimmune diseases (nrAIDs), and healthy controls (HCs). The COVID-19 Vaccination in autoimmune diseases (COVAD) self-reporting e-survey was circulated by a group of > 110 collaborators in 94 countries from March to December 2021. AEs were analyzed between different groups using regression models. Of 10,679 complete respondents [73.8% females, mean age 43 years, 53% Caucasians], 478 had SSc. 83% had completed two vaccine doses, Pfizer-BioNTech (BNT162b2) (51%) was the most common. Minor and major AEs were reported by 81.2% and 3.3% SSc patients, respectively, and did not differ significantly with disease activity or different vaccine types, though with minor symptom differences. Frequencies of AEs were not affected by background immunosuppression, though SSc patients receiving hydroxychloroquine experienced fatigue less commonly (OR 0.4; 95% CI 0.2–0.8). Frequency of AEs and hospitalisations were similar to other AIRDs, nrAIDs, and HC except a higher risk of chills (OR 1.3; 95% CI 1.0–1.7) and fatigue (OR 1.3; 95% CI 1.0–1.6) compared to other AIRDs. COVID-19 vaccines were largely safe and well tolerated in SSc patients in the short term. Background immunosuppression and disease activity did not influence the vaccination-related short-term AEs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00296-023-05310-9.
Keywords: COVID-19, Autoimmune diseases, Vaccination, Systemic sclerosis, Adverse events
Introduction
The healthcare sector across the globe has suffered an unprecedented adverse impact from the COVID-19 pandemic. From a massive uptick in healthcare utilization for an unpredictable new viral infection with potential long-term complications, to disrupting optimal and timely patient evaluations for complex and rare systemic conditions, it has added a substantial mental, social, and economic burden on those with chronic diseases, and rare rheumatic diseases in particular. The prompt development of a multitude of vaccines against SARS-CoV2, many utilizing novel technologies, has singlehandedly improved outcomes including hospitalization risk and mortality following COVID-19 infection. This simultaneously raised concerns on their safety. However, there are now several reports demonstrating the safety and efficacy of COVID-19 vaccination in the general population at large [1]. With the appropriate exclusion of patients with autoimmune rheumatic diseases (AIRDs) from initial vaccine trials given their complex multisystem diseases and often immunosuppressed state, there remained significant gaps in knowledge about vaccine tolerance in these vulnerable populations. Studies utilizing physician-reported registry data have demonstrated the safety and tolerability of COVID-19 vaccination in patients with AIRDs [2, 3]. While recent studies have shown the safety of COVID-19 vaccination in rheumatic diseases like idiopathic inflammatory myositis (IIM) [4], rheumatoid arthritis (RA) [5], and systemic lupus erythematosus (SLE) [6], a significant gap exists in understanding the safety of COVID-19 vaccination in patients with rarer rheumatic diseases such as systemic sclerosis (SSc).
One study reported greater hesitance among patients with SSc in receiving the COVID-19 vaccine than those with other rheumatic diseases [7]. The Scleroderma Patient-centered Intervention Network (SPIN) cohort study details the experiences with COVID-19 vaccines in SSc [8]. The study included 932 respondents, of who 90% had been vaccinated or intended to be vaccinated, and 10% were vaccine-hesitant. 6% of participants made medication changes before their first and 8% before their second vaccine dose. Adverse reactions post-vaccination were similar to that of the general population. Self-reported flares and serious adverse reactions were uncommon. The studies available on vaccine AEs in AIRDs are largely regional and have an under-representation of various ethnic groups (Blacks and Asians) [9, 10].
In this study, we evaluate the short-term safety of COVID-19 vaccination, analyzing the 7-day post-vaccination AEs reported by patients with SSc in comparison to those reported by patients with other AIRDs, non-rheumatic autoimmune diseases (nrAIDs), and healthy controls (HCs) using a patient self-reported global multi-center electronic survey.
Methods
Study design
This study is an international, cross-sectional, multi-center electronic survey, part of the COVAD (COVID-19 Vaccination In Autoimmune Disease) study conducted between March and December 2021 [11]. Informed consent from participants (with and without AIRD) was taken via a cover letter during the survey. Convenience sampling was undertaken. Approval was obtained from the local institutional ethics committee [Sanjay Gandhi Postgraduate Institute of Medical Sciences (2021-143-IP-EXP-39)], and a standardized checklist for reporting the results of the e-survey was followed while reporting [12, 13].
Data collection
A comprehensive patient-self-reporting electronic survey was developed, consisting of a questionnaire of 36 COVID-19 and AIRD-related questions, which included demographic details, AIRD diagnosis, treatment details, current symptom status, COVID-19 vaccination details, 7-day short-term post-vaccination AEs (based on CDC criteria), and patient-reported outcome measures. The survey was pilot-tested and validated in 18 languages by a panel of international collaborators and was hosted on an online platform—surveymonkey.com—and circulated by the international COVAD study group (over 110 physicians) in healthcare centers in over 94 countries (Supplementary Table 1), as well as through numerous social media platforms and online patient support groups. Patients with multiple overlapping autoimmune diseases were put into all the corresponding categories. All participants over the age of 18 years were included. Methods have been detailed in the published COVAD study protocol [11].
Data extraction
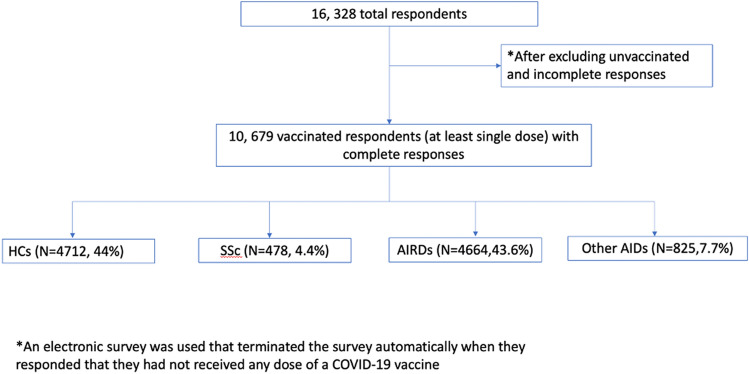
Patients who had not received a single dose of any COVID-19 vaccine at the time of survey completion and who had not completed the survey in full were excluded from the analysis (Fig. 1). Respondents who received at least a single dose of a COVID-19 vaccine and completed the full survey were identified. Multiple relevant variables were extracted from the survey responses of the included participants, including COVID-19 infection history and 7-day post-vaccination AEs.
Fig. 1.
Flow diagram of patients enrolled in the study
Active and inactive disease
Active/Inactive disease was first assessed by the question ‘What was the status of your autoimmune disease four weeks (prior to) before the first dose of COVID-19 vaccine?’ Those who responded as having ‘My disease was inactive or in remission’ were considered to have inactive disease. Those who responded with ‘My disease was active and improving/stable/worsening’ was considered to have active disease. Those who responded to the question as ‘I am not sure or Others (please specify)’ were assessed for disease activity based on 1. The need for stepping up the immunosuppression in the last 6 months or 2. The symptoms they had before immunization (presence of two or more of Raynaud’s phenomenon/fingertips ulcers, shortness of breath, skin thickening, and swallowing difficulty). Those who had either of 1 or 2 were considered to have active disease.
Adverse events post-vaccination
Seven-day AEs were categorized as injection site pain and reaction, minor AEs, major AEs, and hospitalizations. Major AEs consisted of serious reactions to vaccination, requiring urgent medical attention, including anaphylaxis, a marked difficulty in breathing, throat closure (choking), and severe rashes (12). Minor AEs included myalgia, body aches, fever, chills, nausea and vomiting, headache, rashes, fatigue, diarrhea, abdominal pain, high pulse rate or palpitations, rise in blood pressure, fainting, difficulty in breathing, dizziness, and chest pain. Non-listed AEs were reported as “others” as an open-ended question.
Statistical analysis
Chi-square and Mann–Whitney tests were used for categorical and continuous variables, respectively. After univariable analysis, the variables expected to be independently significant between SSc, AIRDs, and HCs underwent binary logistic regression analysis (BLR) with adjustment for factors including age, gender, ethnicity, immunosuppressants, vaccine received, and stratified by country of origin. The results for continuous variables were expressed as median (IQR). P < 0.05 was considered significant. Statistical analysis was performed using IBM SPSS version 26.
Results
Population characteristics of the total cohort
16,328 respondents completed the survey, of whom a total of 10,679 complete respondents were included in the analysis [73.8% females, median aged 43 (30–56) years, 53% Caucasians]. They were primarily from the UK (25%), Italy (24%), Switzerland (8%), India (7%), consisting of 478 (4.4%) SSc patients, 4664 (43.6%) other AIRDs, 4712 (44%) HCs, and 825 (7.7%) nrAIDs. The most common AIRD in this cohort was rheumatoid arthritis (12.6%, N = 1347) followed by SLE (5.5%, N = 582), and the most common nrAIDs were hypo/hyperthyroidism (4.7%, N = 498). All patients received at least one dose of the COVID-19 vaccine and 72.4% received both doses. Pfizer-BioNTech (BNT162b2) was the most common (40.4%, N = 4318) vaccine received, followed by Sinopharm (BBIBP-CorV) (N = 1564, 14.6%) & Oxford/Astra Zeneca (ChAdOx1 nCoV-19) (N = 148,213.9%). Methotrexate (N = 1234, 11.6%) was the most common immunosuppressant used and 15.8% of patients were on glucocorticoids. Other characteristics of the cohort are provided in Table 1.
Table 1.
Baseline characteristics of the cohort
| Variable | Total (n = 10,679) | SSc (n = 478) | HC (N = 4712) | Other AIRD (N = 4664) | nrAIDs (N = 825) |
|---|---|---|---|---|---|
| Age in years; Median (IQR) | 43 (30–56) | 54 (44–64) | 34 (26–47)*** | 50 (38–62) | 42 (33–52)*** |
| Gender | |||||
| Male | 2731 (25.6) | 54 (11.3) | 1655 (35.1) | 916 (19.6) | 107 (13) |
| Female | 7886 (73.8) | 421 (88.1) | 3028 (64.3)*** | 3727 (79.9)*** | 710 (86.1) |
| Do not wish to disclose | 61 (0.6) | 3 (0.6) | 29 (0.6) | 21 (0.5) | 8 (1) |
| No. of doses of vaccine | |||||
| 1 | 2951 (27.6) | 80 (16.7) | 1247 (26.5)*** | 1335 (28.6)*** | 289 (35) |
| 2 | 7728 (72.4) | 398 (83.3) | 3465 (73.5) | 3329 (71.4) | 536 (65) |
| Ethnicity | |||||
| Caucasian | 5662 (53) | 355 (74.3) | 1973 (41.9) | 2852 (61.1) | 482 (58.4) |
| African American or of African origin | 105 (10) | 3 (0.6) | 33 (0.7) | 65 (1.4) | 4 (0.5) |
| Asian | 2608 (24.4) | 70 (14.6) | 1314 (27.9) | 1044 (22.4) | 180 (21.8) |
| Hispanic | 1220 (11.4) | 16 (3.3) | 759 (16.1) | 343 (7.4) | 102 (12.4) |
| Native American/Indigenous/Pacific Islander | 47 (0.4) | 1 (0.2) | 24 (0.5) | 22 (0.5) | – |
| Do not wish to disclose | 576 (5.4) | 13 (2.7) | 345 (7.3) | 188 (4.0) | 30 (3.6) |
| Other | 461 (4.3) | 20 (4.2) | 264 (5.6) | 150 (3.2) | 27 (3.3) |
| Vaccine taken | |||||
| Pfizer-BioNTech (BNT162b2) | 4318 (40.4) | 245 (51.3) | 1598 (33.9) | 2116 (45.4)* | 359 (43.5)* |
| Oxford/Astra Zeneca (ChAdOx1 nCoV-19) | 1482 (13.9) | 111 (23.2) | 483 (10.3)*** | 774 (16.6)*** | 114 (13.8)*** |
| Johnson & Johnson (J&J) (JNJ-78436735) | 99 (0.9) | 2 (0.4) | 37 (0.8) | 46 (1.0) | 14 (1.7)* |
| Moderna (mRNA-1273) | 922 (8.6) | 59 (12.3) | 186 (3.9)*** | 601 (12.9) | 76 (9.2) |
| Novavax (NVX-CoV2373) | 10 (0.1) | - | 5 (0.1) | 4 (0.1) | 1 (0.1) |
| Covishield (ChAdOx1 nCoV-19) | 1177 (11) | 25 (5.2) | 665 (14.1)*** | 391 (8.4)* | 96 (11.6)*** |
| Covaxin (BBV152) | 231 (2.2) | 7 (1.5) | 118 (2.5) | 89 (1.9) | 17 (2.1) |
| Sputnik (Gam-COVID-Vac) | 192 (1.8) | 1 (0.2) | 129 (2.7)** | 49 (1.1) | 13 (1.6)* |
| Sinopharm (BBIBP-CorV) | 1564 (14.6) | 13 (2.7) | 1200 (25.5)*** | 254 (5.4)* | 97 (11.8)*** |
| Others | 626 (5.9) | 13 (2.7) | 256 (5.4) | 321 (6.9)*** | 36 (4.4) |
| Diagnosis | |||||
| No autoimmune disease | 4712 (44.1) | – | – | – | – |
| Rheumatoid arthritis | 1347 (12.6) | 32 (6.6) | – | 1347 (28.9) | – |
| SLE | 583 (5.5) | 19 (3.9) | – | 583 (12.5) | – |
| Systemic sclerosis | 478 (4.4) | 478 (100.0) | – | – | |
| Ankylosing spondylitis or psoriatic arthritis | 372 (3.5) | 0 (0) | – | 372 (8) | – |
| Sjögren’s syndrome | 218 (2.0) | 59 (12.2) | – | 218 (4.7) | – |
| Mixed connective tissue disorder (MCTD) | 142 (1.3) | 24 (4.9) | – | 118 (2.5) | – |
| Vasculitis | 114 (1.1) | 9 (1.8) | – | 114 (2.4) | – |
| Crohn’s disease or ulcerative colitis (IBD) | 175 (1.6) | 5 (1.0) | – | – | 175 (21.2) |
| Thyroid (hypothyroid or hyperthyroid) | 498 (4.7) | 51 (10.5) | – | – | 498 (60.4) |
| Type 1 diabetes | 63 (0.6) | 1 (0.2) | – | – | 63 (7.6) |
| Multiple sclerosis | 31 (0.3) | 1 (0.2) | – | – | 31 (3.6) |
| Myasthenia gravis | 30 (0.3) | 0 (0) | – | – | 30 (3.6) |
| Pernicious anemia | 10 (0.1) | 2 (0.4) | – | – | 18 (2.2) |
| Hemolytic anemia/ITP | 18 (0.2) | 0 (0) | – | – | 18 (2.2) |
| Polymyalgia rheumatica | 18 (0.2) | 1 (0.2) | – | 18 (0.4) | – |
| Dermatomyositis | 478 (4.4) | 15 (3.1) | – | 478 (4.4) | – |
| Polymyositis | 278 (2.6) | 23 (4.7) | – | 278 (2.6) | – |
| Others | 724 (6.8) | 47 (9.7) | – | 724 (15.5) | – |
| Treatment | |||||
| Any immunosuppressants | 8707 (81.5) | 311 (65) | – | 3386 (72.5)** | 298 (36)*** |
| Methotrexate | 1234 (11.6) | 63 (13.2) | – | 1147 (24.6)*** | 24 (2.9)*** |
| MMF | 558 (5.2) | 123 (25.7) | – | 420 (9)*** | 15 (1.8)*** |
| Rituximab | 97 (0.9) | 7 (1.5) | – | 87 (1.9) | 3 (0.4) |
| Azathioprine | 379 (3.5) | 21 (4.4) | – | 311 (6.7) | 47 (5.7) |
| Hydroxychloroquine | 1176 (11) | 88 (18.4) | – | 1073 (23)* | 15 (1.8)*** |
| Sulfasalazine | 257 (2.4) | – | – | 241 (5.2) | 16 (1.9) |
| Leflunomide | 175 (1.6) | 1 (0.2) | – | 172 (3.7)*** | 2 (0.2) |
| Oral tacrolimus | 73 (0.7) | 4 (0.8) | – | 66 (1.4) | 3 (0.4) |
| Cyclosporine | 59 (0.6) | – | – | 56 (1.2) | 3 (0.4) |
| IVIG | 169 (1.6) | 8 (1.7) | – | 150 (3.2)*** | 11 (1.3) |
| Cyclophosphamide | 19 (0.2) | 5 (1.0) | – | 14 (0.3) | – |
| Any steroid dose | 1693 (15.8) | 114 (23.6) | – | 1493 (32)* | 86 (10.4)* |
| No steroids | 4272 (40) | 363 (75.9) | – | 3170 (68) | 739 (89.6) |
| < 10 mg steroids | 1318 (12.3) | 100 (20.9) | – | 1164 (25) | 54 (6.5) |
| 10–20 mg steroids | 269 (2.5) | 12 (2.5) | – | 239 (5.1) | 18 (2.2) |
| > 20 mg steroids | 106 (1) | 2 (0.4) | – | 90 (1.9) | 14 (1.7) |
AIRDs Autoimmune rheumatic disease, nrAID non-rheumatic autoimmune disease, HC healthy controls, SSc systemic sclerosis
*p < 0.05, **p < 0.005, ***p < 0.001. Comparisons are made between SSc and other groups respectively. Chi-square for categorical variables, Mann–Whitney U for scale variables
Population characteristics of SSc patients
Out of 10,679 respondents, SSc patients constituted 4.4%, with a median age of 54 (44–64) years and 88% females. Caucasians were the most common respondents (74%), followed by Asians (14.6%). 83% of SSc patients received both doses of the vaccine. Pfizer-BioNTech (BNT162b2) was the most common vaccine received (51%), followed by Oxford/Astra Zeneca (ChAdOx1 nCoV-19) (23%) and Moderna (mRNA-1273) (12%).
Patients with SSc were older [54 (44–64) years] than HCs [34(26–47), p < 0.001] and nrAIDs [42 (33–52), p < 0.001] with higher Caucasian representation (74% in SSc; 61% in other AIRDs; 41% in HCs) and higher Pfizer-BioNTech (BNT162b2) vaccine received (51% in SSc as compared to 45% in other AIRDs and 34% in HCs). Fewer patients were on immunosuppressants in SSc (65%) as compared to other AIRDS (72%). MMF was the most common immunosuppressant received in SSc patients (25%), followed by hydroxychloroquine (18%) and methotrexate (13%). Glucocorticoids were being received by 23.6% (Table 1).
Post-COVID-19 vaccination-related AEs in SSc patients
Any AEs were reported by 388 (81.2%) respondents, all were minor AEs. Any major AE was reported by 16 (3.3%) SSc patients. Injection site pain was the commonest AE reported by 319 (66.7%) respondents. Other minor AEs reported were fatigue (37%), headache (26%), body aches (19.9%), fever (19.7%), chills (18%), myalgia (15.9%), nausea/vomiting (7%), and others 8%. Marked dyspnea was reported in 3 (0.6%), throat closure in 1 (0.2%), and severe rashes in 1 (0.2%). None of the recipients had anaphylaxis. All-cause hospitalization was seen in 3 patients (0.6%) (Supplementary table 2).
Post-COVID-19 vaccination-related AEs among the SSc patients with self-reported active and inactive disease are presented in Table 2. Among the 478 SSc patients, 375 had active SSc 4 weeks before vaccination, and 102 had inactive disease. Vaccine-related AEs were comparable between those groups. Hospitalization rates were also similar.
Table 2.
Comparison of vaccination-related AE among Active and Inactive SSc
| N (%) | Active SSc (n = 375) | Inactive SSc (n = 102) | Univariate | Multivariable | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |||
| Any AE | 257 (80.6) | 73 (83.9) | – | 0.478 | – | – |
| Injection site pain | 253 (67.5) | 65 (63.7) | – | 0.477 | – | – |
| Minor AEs | ||||||
| Any minor AEs | 257 (80.6) | 73 (83.9) | – | 0.478 | – | – |
| Myalgia | 58 (15.5) | 18 (17.6) | – | 0.594 | – | – |
| Body ache | 74 (19.7) | 21 (20.6) | – | 0.848 | – | – |
| Fever | 76 (20.3) | 17 (16.7) | – | 0.416 | – | – |
| Chills | 72 (19.2) | 15 (14.7) | – | 0.297 | – | – |
| Nausea and vomiting | 30 (8) | 6 (5.9) | – | 0.473 | – | – |
| Headache | 102 (27.2) | 22 (21.6) | – | 0.25 | – | – |
| Rashes | 8 (2.1) | 5 (4.9) | – | 0.128 | – | – |
| Fatigue | 148 (39.5) | 31 (30.4) | – | 0.093 | – | – |
| Diarrhea | 12 (3.2) | 5 (4.9) | – | 0.411 | – | – |
| Abdominal pain | 9 (2.4) | 2 (2) | – | 0.793 | – | – |
| Palpitations | 9 (2.4) | 7 (6.9) | 0.3 (0.1–0.9) | 0.026 | 0.3 (0.1–1.0) | 0.064 |
| Rise in blood pressure | 4 (1.1) | 1 (1) | – | 0.94 | – | – |
| Fainting | 2 (0.5) | 2 (2) | – | 0.161 | – | – |
| Difficulty in breathing | 5 (1.3) | 1 (1) | – | 0.777 | – | – |
| Dizziness | 23 (6.1) | 4 (3.9) | – | 0.391 | – | – |
| Chest pain | 4 (1.1) | 0 (0) | – | 0.295 | – | – |
| Others | 31 (9.7) | 6 (6.9) | – | 0.418 | – | – |
| Major AEs | ||||||
| Any major AE | 9 (2.8) | 4 (4.6) | – | 0.404 | – | – |
| Anaphylaxis | 0 (0) | 0 (0) | – | – | – | – |
| Marked dyspnea | 3 (0.8) | 0 (0) | – | 0.365 | – | – |
| Throat closure | 1 (0.3) | 0 (0) | – | 0.602 | – | – |
| Severe rashes | 1 (0.3) | 0 (0) | – | 0.602 | – | – |
| Others | 6 (1.9) | 4 (4.6) | – | 0.147 | – | – |
| Hospitalization | 2 (0.5) | 1 (1) | – | 0.613 | – | – |
SSc systemic sclerosis, adverse event, OR odd’s ratio, CI confidence interval
Chi-square for categorical variables and Mann–Whitney test for continuous variables
Factors adjusted in multivariable analysis (binary logistic regression) include age, gender, ethnicity, country by income
Vaccination-related AEs among SSc patients based on the type of vaccine received are presented in Table 3 and Supplementary table 3. The overall frequency of AEs, minor AEs, major AEs, and hospitalisations was similar between the recipients of different vaccines except for a few differences in selected minor AEs. Pfizer-BioNTech (BNT162b2) vaccine recipients reported a lower frequency of body aches, fever, chills, nausea/vomiting than the rest of the vaccine recipients (OR ranging between 0.2 and 0.4). Oxford/Astra Zeneca (ChAdOx1 nCoV-19) receivers reported a higher frequency of body aches, fever, chills, nausea/vomiting, headache, and fatigue compared to the rest of the vaccine recipients (OR ranging between 2.0 and 5.1). Similarly, Moderna (mRNA-1273) vaccine recipients reported a higher frequency of fever, chills, and chest pain compared to the rest (OR ranging between 2.6 and 8.9). Covishield (Serum Institute India) (ChAdOx1 nCoV-19) vaccine recipients reported a higher frequency of body aches and fever compared to the rest of the vaccine recipients (OR ranging between 3.4 and 4.6). Sinopharm (BBIBP-CorV) vaccine recipients reported lower injection site pain frequency than the rest (OR 0.2; 95% CI 0.06–0.7).
Table 3.
Comparison of vaccination AEs according to the vaccine received in SSc patients (n = 487)
| N (%) | Pfizer-BioNTech (BNT162b2) (N = 216) | Oxford/Astra Zeneca (ChAdOx1 nCoV-19) (N = 106) | Moderna (mRNA-1273) (N = 38) | Covishield (Serum Institute India) (ChAdOx1 nCoV-19) (N = 14) | Sinopharm (BBIBP-CorV) (N = 9) |
|---|---|---|---|---|---|
| Any adverse effect | 170 (78.7) | 94 (88.7) | 3 (86.8) | 10 (71.4) | 6 (66.7) |
| Injection site pain | 169 (69) | 74 (66.7) | 45 (76.3) | 16 (64) | 5 (38.5) |
| Minor AEs | |||||
| Any minor AE | 170 (78.7) | 94 (88.7) | 33 (86.8) | 10 (71.4) | 6 (66.7) |
| Myalgia | 37 (15.1) | 25 (22.5) | 10 (16.9) | 2 (8) | 1 (7.7) |
| Body ache | 35 (14.3) | 38 (34.2) | 11 (18.6) | 10 (40) | 0 (0) |
| Fever | 27 (11) | 31 (27.9) | 18 (30.5) | 13 (52) | 3 (23.1) |
| Chills | 28 (11.4) | 35 (31.5) | 18 (30.5) | 4 (16) | 1 (7.7) |
| Nausea and vomiting | 10 (4.1) | 18 (16.25) | 5 (8.5) | 1 (4) | 0 (0) |
| Headache | 56 (22.9) | 43 (38.7) | 19 (32.2) | 4 (16) | 1 (7.7) |
| Rashes | 4 (1.6) | 3 (2.7) | 5 (8.5) | 1 (4) | 0 (0) |
| Fatigue | 86 (35.1) | 56 (50.5) | 26 (44.1) | 11 (44) | 0 (0) |
| Diarrhea | 10 (4.1) | 2 (1.8) | 4 (6.8) | 0 (0) | 1 (7.7) |
| Abdominal pain | 5 (2.0) | 3 (2.7) | 3 (5.1) | 0 (0) | 0 (0) |
| High pulse rate | 7 (2.9) | 2 (1.8) | 5 (8.5) | 0 (0) | 0 (0) |
| Rise in blood pressure | 3 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fainting | 3 (1.2) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Difficulty in breathing | 2 (0.8) | 3 (2.7) | 0 (0) | 0 (0) | 0 (0) |
| Dizziness | 9 (3.7) | 9 (8.1) | 6 (10.2) | 3 (12) | 0 (0) |
| Chest pain | 2 (08) | 0 (0) | 2 (3.4) | 0 (0) | 0 (0) |
| Others | 17 (7.9) | 10 (9.4) | 5 (13.2) | 1 (7.1) | 2 (22.2) |
| Major AEs | |||||
| Any major AE | 7 (3.2) | 2 (1.9) | 2 (5,3) | 1 (7.1) | 0 (0) |
| Anaphylaxis | 5 (2.3) | 2 (1.9) | 2 (5.3) | 0 (0) | 0 (0) |
| Marked dyspnea | 1 (0.4) | 0 (0) | 0 (0) | 1 (4) | 0 (0) |
| Throat closure | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 0 (0) |
| Severe rashes | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Others | 5 (2.3) | 2 (1.9) | 2 (5.3) | 0 (0) | 0 (0) |
| Hospitalization | 0 (0) | 0 (0) | 1 (1.7) | 2 (8) | 0(0) |
AE Adverse events SSc Systemic sclerosis
Chi-square for categorical variables and Mann–Whitney test for Scale variables
Comparisons are between one vaccine type versus rest, BOLD have increased OR when compared to rest, BOLD Underlined have decreased OR when compared to rest in binary logistic regression
Vaccination-related AEs among SSc based on the background immunosuppression are presented in Table 4 and supplementary Table 4. Overall, the frequency of vaccination-related AEs and hospitalisations was similar irrespective of the background immunosuppression. However, SSc patients on hydroxychloroquine reported lower fatigue (OR 0.4; 95% CI 0.2–0.8; p = 0.013) compared to those not on hydroxychloroquine.
Table 4.
Vaccine AEs among SSc patients based on the immunosuppression received (n = 487)
| N (%) | Methotrexate | Mycophenolate mofetil | Hydroxychloroquine | Azathioprine | Glucocorticoids < 10 mg |
|---|---|---|---|---|---|
| (N = 51) | (N = 110) | (N = 82) | (N = 18) | (N = 98) | |
| Any adverse effect | 43 (84.3) | 86 (78.2) | 68 (82.9) | 15 (83.3) | 75 (76.5) |
| Injection site pain | 46 (73) | 87 (70.7) | 67 (76.1) | 13 (61.9) | 78 (68.4) |
| Any minor AE | 43 (84.3) | 86 (78.2) | 68 (82.9) | 15 (83.3) | 75 (76.5) |
| Myalgia | 12 (19) | 15 (12.2) | 12 (13.6) | 4 (19) | 19 (16.7) |
| Body ache | 15 (23.8) | 25 (20.3) | 17 (19.3) | 2 (9.5) | 21 (18.4) |
| Fever | 10 (15.9) | 26 (21.1) | 18 (20.5) | 4 (19) | 22 (19.3) |
| Chills | 15 (23.8) | 20 (16.3) | 11 (12.5) | 3 (14.3) | 19 (16.7) |
| Nausea and vomiting | 7 (11.1) | 10 (8.1) | 6 (6.8) | 4 (19) | 11 (9.6) |
| Headache | 16 (25.4) | 33 (26.8) | 22 (25) | 6 (28.6) | 23 (20.2) |
| Rashes | 3 (4.8) | 1 (0.8) | 0 (0) | 0 (0) | 2 (1.8) |
| Fatigue | 26 (41.3) | 42 (34.1) | 24 (27.3) | 6 (28.6) | 37 (32.5) |
| Diarrhea | 2 (3.2) | 2 (1.6) | 2 (2.3) | 0 (0) | 4 (4.4) |
| Abdominal pain | 0 (0) | 2 (1.6) | 2 (2.3) | 0 (0) | 2 (1.8) |
| High pulse rate | 3 (4.8) | 3 (2.4) | 3 (3.4) | 0 (0) | 4 (3.5) |
| Rise in blood pressure | 1 (1.6) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) |
| Fainting | 0 (0) | 1 (0.8) | 1 (1.1) | 0 (0) | 1 (0.9) |
| Difficulty in breathing | 1 (1.6) | 2 (1.6) | 1 (1.1) | 0 (0) | 3 (2.6) |
| Dizziness | 3 (4.8) | 6 (4.9) | 4 (4.5) | 3 (14.3) | 7 (6.1) |
| Chest pain | 0 (0) | 1 (0.8) | 1 (1.1) | 1 (4.8) | 1 (0.9) |
| Others | 4 (7.8) | 12 (10.9) | 10 (12.2) | 2 (11.1) | 13 (13.3) |
| Any Major AE | 1 (2) | 3 (2.7) | 3 (3.7) | 2 (11.1) | 2 (2) |
| Anaphylaxis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Marked dyspnea | 0 (0) | 0 (0) | 1 (1.1) | 0 (0) | 1 (0.9) |
| Throat closure | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Severe rashes | 1 (1.6) | 0 (0) | 1 (1.1) | 0 (0) | 1 (0.9) |
| Others | 1 (2) | 2 (1.8) | 2 (2.4) | 2 (11.1) | 0 (0) |
| Hospitalization | 1 (1.6) | 0 (0) | 1 (1.1) | 0 (0) | 0 (0) |
AE Adverse events SSc Systemic sclerosis
Chi-square for categorical variables and Mann–Whitney test for Scale variables
Comparisons are between one vaccine type versus rest, BOLD have increased OR when compared to rest, BOLD Underlined have decreased OR when compared to rest in binary logistic regression
Comparison of vaccination-related AEs between SSc, other AIRDs, other AIDs, and HCs
Vaccination-related AEs between SSc, HCs, AIRDs, and nrAIDs are presented in Supplementary table 2. The overall frequency of AEs, minor AEs, major AEs, and hospitalisations was similar between the groups. Compared to other AIRDs, SSc patients had higher chills (adjusted OR 1.3; 95% CI 1.0–1.7; p = 0.049), and fatigue (adjusted OR 1.3; 95% CI 1.0–1.6; p = 0.006).
Discussion
This large global study demonstrates the safety and tolerability of COVID-19 vaccination in patients with SSc, a vulnerable group with a high prevalence of cardiopulmonary comorbidity, often treated with immune suppression. The majority of vaccine-related AEs experienced by SSc patients were minor, without any increased risk of major AEs or hospitalizations in comparison with HCs and other AIRDs. AEs were also similar irrespective of disease activity and background immunosuppression. This study adds to the slowly building evidence for the benefits of COVID-19 vaccination outweighing the risk of post-vaccination AEs in patients with rheumatic diseases. It provides a boost to guidance statements by the American College of Rheumatology that encourage COVID-19 vaccination in patients with rheumatic diseases [14].
Large-scale studies on the safety of COVID-19 vaccination in SSc patients are lacking. Our study reported an overall frequency of AEs in 81.2%, which is higher than previously reported studies in SSc (39%) patients [8] and patients with systemic rheumatic diseases [10]. In the SPIN cohort study, 39% and 58% had adverse reactions after the first and second doses of vaccination, respectively [8]. Reassuringly, the patients with SSc had similar frequencies of AEs and hospitalization compared to HCs and other AIRDs.
Among the vaccine types, this study demonstrated that SSc patients have lower AEs with Pfizer-BioNTech and higher systemic AEs with Oxford/Astra Zeneca and Moderna compared with other vaccines. These results were consistent with the meta-analysis of twenty-seven studies and twenty-four case reports which overviewed the side effects and adverse clinical cases reported after COVID-19 immunization in a healthy population [15]. In the SPIN cohort study, higher AEs reported for Astra Zeneca and Moderna vaccines compared with the Pfizer vaccine after the first and second doses of vaccination were also similar to the present study. These differences in AEs among vaccines could be due to different adjuvants, their interaction with underlying immune dysfunction and immunosuppressive medications, and different post-manufacturing processes [16].
Overall, AEs were similar for active and inactive SSc patients, similar to RA, and SLE patients in the COVAD cohort [17], and unlike IIM patients who had higher AEs among active disease [4]. The frequency of vaccine-related AEs and hospitalization were also similar irrespective of background immunosuppression. To our knowledge, this is the first study comparing vaccine-related AEs in SSc patients accounting for background immunosuppression and self-reported disease activity.
The study has several limitations inherent to the patient self-reported electronic survey design. The data cannot be verified by medical records and is affected by recall bias. The survey focused on seven days post-vaccination AEs; long-term outcomes and disease flares post-vaccination were not assessed in the present study. However, a second survey encompassing long-term AEs and breakthrough infections in this study population is currently underway and will be very informative [18].
The cohort sampled patients by convenience sampling. Those patients without internet or smartphone access, those with lower socioeconomic status, disability, or physical inabilities such as those with contractures and digital amputations, and deceased patients are not well represented in the cohort. African and Afro-American ethnicity are also underrepresented in the cohort, although other ethnic representations were adequate. The definition of active and inactive disease used in the study gages patient self-reported assessment of their disease activity but is arbitrary, and akin to the patient global assessment of disease but not a validated patient-reported outcome. Granularity on disease characteristics of SSc (diffuse vs limited skin phenotype, presence and severity of ILD or prevalence of pulmonary arterial hypertension, serology) was not captured in this study, and stratification of vaccine safety by SSc disease subsets cannot be addressed by this study.
The strengths of the study are a large sample size, with the global representation of many different ethnicities, and a wide representation of rare AIRDs like systemic sclerosis besides other nrAIDs.
Conclusions
Our study adds to the growing body of literature supporting the safety of COVID-19 vaccination in patients with rarer, complex systemic autoimmune diseases. It showed a favorable short-term safety profile in SSc patients irrespective of self-reported disease activity and vaccine types received, with vaccination-related AEs similar to healthy controls. Studies to evaluate the long-term outcomes, efficacy, and disease flares following vaccination remain an unmet need for developing future COVID-19 vaccination guidelines in patients with SSc.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to all respondents for completing the questionnaire. The authors also thank the Myositis Association, Myositis India, Myositis UK, Myositis Support and Understanding, the Myositis Global Network, Deutsche Gesellschaft für Muskelkranke e.V. (DGM), Dutch and Swedish Myositis patient support groups, Cure JM, Cure IBM, Sjögren’s India Foundation, Patients Engage, Scleroderma India, Lupus UK, Lupus Sweden, Emirates Arthritis Foundation, EULAR PARE, ArLAR research group, AAAA patient group, Myositis Association of Australia, APLAR myositis special interest group, Thai Rheumatism association, PANLAR, AFLAR NRAS, Anti-Synthetase Syndrome support group, and various other patient support groups and organizations for their contribution to the dissemination of this survey. Finally, the authors wish to thank all members of the COVAD study group for their invaluable role in the data collection.
COVAD Study Group authors: Bhupen Barman, Yogesh Preet Singh, Rajiv Ranjan, Avinash Jain, Sapan C Pandya, Rakesh Kumar Pilania, Aman Sharma, Manesh Manoj M, Vikas Gupta, Chengappa G Kavadichanda, Pradeepta Sekhar Patro, Sajal Ajmani, Sanat Phatak, Rudra Prosad Goswami, Abhra Chandra Chowdhury, Ashish Jacob Mathew, Padnamabha Shenoy, Ajay Asranna, Keerthi Talari Bommakanti, Anuj Shukla, Arunkumar R Pande, Kunal Chandwar, Döndü Üsküdar Cansu, John D Pauling, Chris Wincup, Ashima Makol, Nicoletta Del Papa, Gianluca Sambataro, Atzeni Fabiola, Marcello Govoni, Simone Parisi, Elena Bartoloni Bocci, Gian Domenico Sebastiani, Enrico Fusaro, Marco Sebastiani, Luca Quartuccio, Franco Franceschini, Pier Paolo Sainaghi, Giovanni Orsolini, Rossella De Angelis, Maria Giovanna Danielli, Vincenzo Venerito, Lisa S Traboco, Suryo Anggoro Kusumo Wibowo, Jorge Rojas Serrano, Ignacio García-De La Torre, Erick Adrian Zamora Tehozol, Jesús Loarce-Martos, Sergio Prieto-González, Raquel Aranega Gonzalez, Akira Yoshida, Ran Nakashima, Shinji Sato, Naoki Kimura, Yuko Kaneko, Stylianos Tomaras, Margarita Aleksandrovna Gromova, Or Aharonov, Ihsane Hmamouchi, Leonardo Santos Hoff, Margherita Giannini, François Maurier, Julien Campagne, Alain Meyer, Melinda Nagy-Vincze, Daman Langguth, Vidya Limaye, Merrilee Needham, Nilesh Srivastav, Marie Hudson, Océane Landon-Cardinal, Syahrul Sazliyana Shaharir, Wilmer Gerardo Rojas Zuleta, José António Pereira Silva, João Eurico Fonseca, Olena Zimba
Author contributions
Conceptualisation: AM, NR, DT, VA, and LG. Data curation: All authors. Formal analysis: NR; Funding acquisition: N/A. Investigation: LG, AM, NR, and DT. Methodology: LG, VA, and NR; Software: LG. Validation: VA, RA, JBL, and HC. Visualization: RA, VA, and LG. Writing original draft: DT, NR. Writing–review and editing: all authors.
Funding
HC was supported by the National Institution for Health Research Manchester Biomedical Research Centre Funding Scheme. The views expressed in this publication are those of the authors and not necessarily those of the NHS, National Institute for Health Research, or Department of Health.
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
ALT has received honoraria for advisory boards and speaking for Abbvie, Gilead, Janssen, Lilly, Novartis, Pfizer, and UCB. EN has received speaker honoraria/participated in advisory boards for Celltrion, Pfizer, Sanofi, Gilead, Galapagos, AbbVie, and Lilly, and holds research grants from Pfizer and Lilly. HC has received grant support from Eli Lilly and UCB, consulting fees from Novartis, Eli Lilly, Orphazyme, Astra Zeneca, speaker for UCB, and Biogen. IP has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Elli Lilly and Company, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Novartis and F. Hoffmann-La Roche AG. JBL has received speaker honoraria/participated in advisory boards for Sanofi Genzyme, Roche, and Biogen. None is related to this manuscript. JD has received research funding from CSL Limited. NZ has received speaker fees, advisory board fees, and research grants from Pfizer, Roche, Abbvie, Eli Lilly, NewBridge, Sanofi-Aventis, Boehringer Ingelheim, Janssen, and Pierre Fabre; none are related to this manuscript. OD has/had consultancy relationship with and/or has received research funding from and/or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three calendar years: 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant, Amgen, AnaMar, Arxx, AstraZeneca, Baecon, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, iQvia, Horizon, Inventiva, Janssen, Kymera, Lupin, Medscape, Merck, Miltenyi Biotec, Mitsubishi Tanabe, Novartis, Prometheus, Redxpharma, Roivant, Sanofi and Topadur. Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143). RA has a consultancy relationship with and/or has received research funding from the following companies: Bristol Myers-Squibb, Pfizer, Genentech, Octapharma, CSL Behring, Mallinckrodt, AstraZeneca, Corbus, Kezar, Abbvie, Janssen, Kyverna Alexion, Argenx, Q32, EMD-Serono, Boehringer Ingelheim, Roivant, Merck, Galapagos, Actigraph, Scipher, Horizon Therepeutics, Teva, Beigene, ANI Pharmaceuticals, Biogen, Nuvig, Capella Bioscience, and CabalettaBio. Rest of the authors have no conflict of interest relevant to this manuscript.
Ethical approval
Ethical approval was obtained from the Institutional Ethics Committee of the Sanjay Gandhi Postgraduate Institute of Medical Sciences, Raebareli Road, Lucknow, 226014.
Footnotes
Vikas Agarwal and Ashima Makol have contributed equally to this work.
The complete list of authors part of the COVAD Study Group as well as their affiliations are provided in the Acknowledgments and Supplementary Material.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ashima Makol, Email: Makol.ashima@mayo.edu.
COVAD Study Group:
Bhupen Barman, Yogesh Preet Singh, Rajiv Ranjan, Avinash Jain, Sapan C. Pandya, Rakesh Kumar Pilania, Aman Sharma, Manesh Manoj M, Vikas Gupta, Chengappa G. Kavadichanda, Pradeepta Sekhar Patro, Sajal Ajmani, Sanat Phatak, Rudra Prosad Goswami, Abhra Chandra Chowdhury, Ashish Jacob Mathew, Padnamabha Shenoy, Ajay Asranna, Keerthi Talari Bommakanti, Anuj Shukla, Arunkumar R. Pande, Kunal Chandwar, Döndü Üsküdar Cansu, John D. Pauling, Chris Wincup, Ashima Makol, Nicoletta Del Papa, Gianluca Sambataro, Atzeni Fabiola, Marcello Govoni, Simone Parisi, Elena Bartoloni Bocci, Gian Domenico Sebastiani, Enrico Fusaro, Marco Sebastiani, Luca Quartuccio, Franco Franceschini, Pier Paolo Sainaghi, Giovanni Orsolini, Rossella De Angelis, Maria Giovanna Danielli, Vincenzo Venerito, Lisa S. Traboco, Suryo Anggoro Kusumo Wibowo, Jorge Rojas Serrano, Ignacio García-De La Torre, Erick Adrian Zamora Tehozol, Jesús Loarce-Martos, Sergio Prieto-González, Raquel Aranega Gonzalez, Akira Yoshida, Ran Nakashima, Shinji Sato, Naoki Kimura, Yuko Kaneko, Stylianos Tomaras, Margarita Aleksandrovna Gromova, Or Aharonov, Ihsane Hmamouchi, Leonardo Santos Hoff, Margherita Giannini, François Maurier, Julien Campagne, Alain Meyer, Melinda Nagy-Vincze, Daman Langguth, Vidya Limaye, Merrilee Needham, Nilesh Srivastav, Marie Hudson, Océane Landon-Cardinal, Syahrul Sazliyana Shaharir, Wilmer Gerardo Rojas Zuleta, José António Pereira Silva, João Eurico Fonseca, and Olena Zimba
References
- 1.Moreira ED, Kitchin N, Xu X, et al. Safety and efficacy of a third dose of BNT162b2 COVID-19 vaccine. N Engl J Med. 2022;386:1910–1921. doi: 10.1056/NEJMoa2200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machado PM, Lawson-Tovey S, Strangfeld A, et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann Rheum Dis. 2022;81:695–709. doi: 10.1136/annrheumdis-2021-221490. [DOI] [PubMed] [Google Scholar]
- 3.Papagoras C, Fragoulis GE, Zioga N, et al. Better outcomes of COVID-19 in vaccinated compared to unvaccinated patients with systemic rheumatic diseases. Ann Rheum Dis. 2022;81:1013–1016. doi: 10.1136/annrheumdis-2021-221539. [DOI] [PubMed] [Google Scholar]
- 4.Gil-Vila A, Naveen R, Selva-O’Callaghan A, et al. COVID-19 vaccination in autoimmune diseases (COVAD) study: vaccine safety in idiopathic inflammatory myopathies. Muscle Nerve. 2022 doi: 10.1002/mus.27681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Tong X, Yeung WWY, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2022;81:564–568. doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.So H, Li T, Chan V, et al. Immunogenicity and safety of inactivated and mRNA COVID-19 vaccines in patients with systemic lupus erythematosus. Ther Adv Musculoskelet Dis. 2022 doi: 10.1177/1759720X221089586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciaffi J, Giuggioli D, Mari A, et al. COVID-19 vaccine hesitancy in systemic sclerosis. Clin Exp Rheumatol. 2021;39(Suppl 131):165–166. doi: 10.55563/clinexprheumatol/vv61xv. [DOI] [PubMed] [Google Scholar]
- 8.Gordon JK, Showalter K, Wu Y, et al. Systemic sclerosis and COVID-19 vaccines: a SPIN Cohort study. Lancet Rheumatol. 2022;4:e243–e246. doi: 10.1016/S2665-9913(21)00416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Y, Geng Y, Wang Y, et al. Safety and disease flare of autoimmune inflammatory rheumatic diseases: a large real-world survey on inactivated COVID-19 vaccines. Ann Rheum Dis. 2022;81:443–445. doi: 10.1136/annrheumdis-2021-221736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sattui SE, Liew JW, Kennedy K, et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2021;7:e001814. doi: 10.1136/rmdopen-2021-001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sen P, Gupta L, Lilleker JB, et al. COVID-19 vaccination in autoimmune disease (COVAD) survey protocol. Rheumatol Int. 2022;42:23–29. doi: 10.1007/s00296-021-05046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet E-surveys (CHERRIES) J Med Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Understanding Adverse Events and Side Effects (2021) | Vaccine Safety | CDC. https://www.cdc.gov/vaccinesafety/ensuringsafety/sideeffects/index.html. Accessed 7 Jan 2022
- 14.Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 1. Arthritis Rheumatol. 2021;73:1093–1107. doi: 10.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabail R, Ahmed W, Ilyas M, et al. The side effects and adverse clinical cases reported after COVID-19 immunization. Vaccines (Basel) 2022;10:488. doi: 10.3390/vaccines10040488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. Npj Vaccines. 2019;4:39. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naveen R, Parodis I, Joshi M, et al. COVID-19 vaccination in autoimmune diseases (COVAD) Study: vaccine safety and tolerance in rheumatoid arthritis. Rheumatology (Oxford) 2022 doi: 10.1093/rheumatology/keac624. [DOI] [PubMed] [Google Scholar]
- 18.Fazal ZZ, Sen P, Joshi M, et al. COVAD survey 2 long-term outcomes: unmet need and protocol. Rheumatol Int. 2022 doi: 10.1007/s00296-022-05157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.