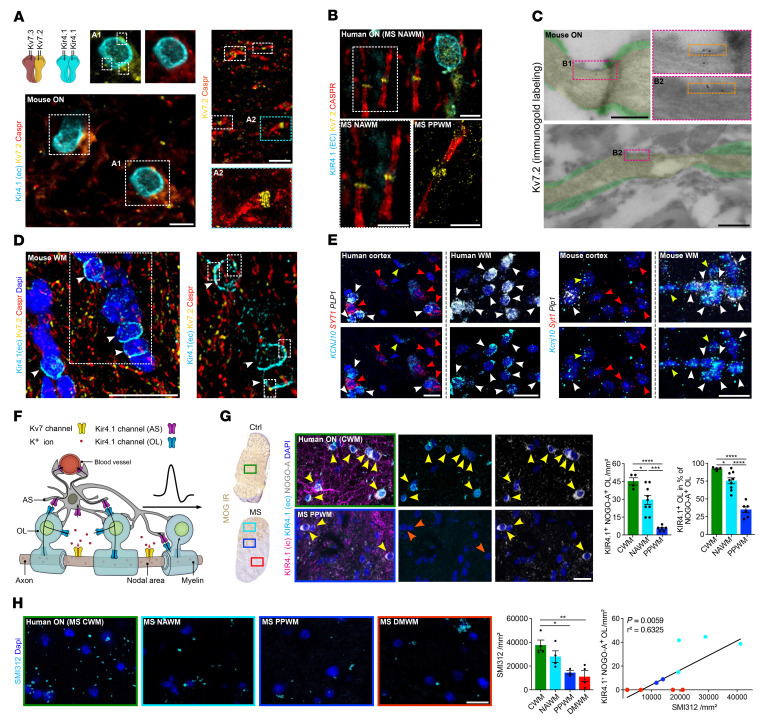
Figure 1. Investigation of Kir4.1 and Kv7 channels in neuroglial cell types under homeostatic and inflammatory demyelinating conditions.
(A and B) Triple staining (Caspr, Kir4.1 [extracellular epitope, EC], Kv7.2) revealed specific nodal expression of Kv7.2 (flanked by Caspr IR) adjacent to OL-Kir4.1 channel IR in mouse (A) and human (B) ON. A2 close-up (stimulated emission depletion [STED] image) shows the approximately 190 nm periodic organization of Kv7.2. (C) Kv7.2 immunogold EM labeling shows the presence of gold particles in nodal areas (yellow) between myelin sheets (green) in control mouse ON. (D) Triple staining (compare with A) confirmed the juxtapositioning of OL-Kir4.1 and nodal Kv7.2 channels (white arrows) in other mouse WM tracts (corpus callosum). (E) Perineuronal mouse Kcnj10 and human KCNJ10 expression (ISH) was visualized in mouse and human cortex with OLs coexpressing Plp1+ (mouse) or PLP1+ (human) and Kcnj10+ or KCNJ10+ (white arrowheads) next to Syt1+, SYT1+, Kcnj10–, and KCNJ10– neurons (red arrowheads). Yellow arrowheads indicate Kcnj10+, KCNJ10+, Plp1–, and PLP1– astrocytes. (F) Cartoon illustrates neuron-OL for the K+ shuttling mechanism: neuronal Kv7 channels mediate axonal K+ efflux, and OL-Kir4.1 channels mediate extracellular K+ uptake and siphoning through interaction with astrocyte (AS) Kir4.1 channels. (G) In human MS ON, KIR4.1 channel IR (antibodies against intracellular [specific for OL-KIR4.1 and AS-KIR4.1] and extracellular [specific for OL-KIR4.1] epitopes) was preserved on astrocyte fibers in lesions. OL-KIR4.1 channel IR (yellow arrows) was reduced in MS NAWM areas (n = 9) and lost in PPWM (n = 6) relative to CWM (n = 4) based on MOG IR. (H) SMI312+ axon density was gradually lost in MS ON tissues toward the lesion rim and correlated with OL-KIR4.1 channel loss. Scale bars: 5 μm (A and B); 0.5 μm (C); 20 μm (D and E, and G); 100 μm (H). Original magnification, ×100 (enlarged insets in A and B) and ×63 (enlarged insets in D). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, by 1-way ANOVA (G and H, left) and simple linear regression (H, right).