Fig. 5 |. GMP-compatible process for nonviral CAR-T cell manufacturing.
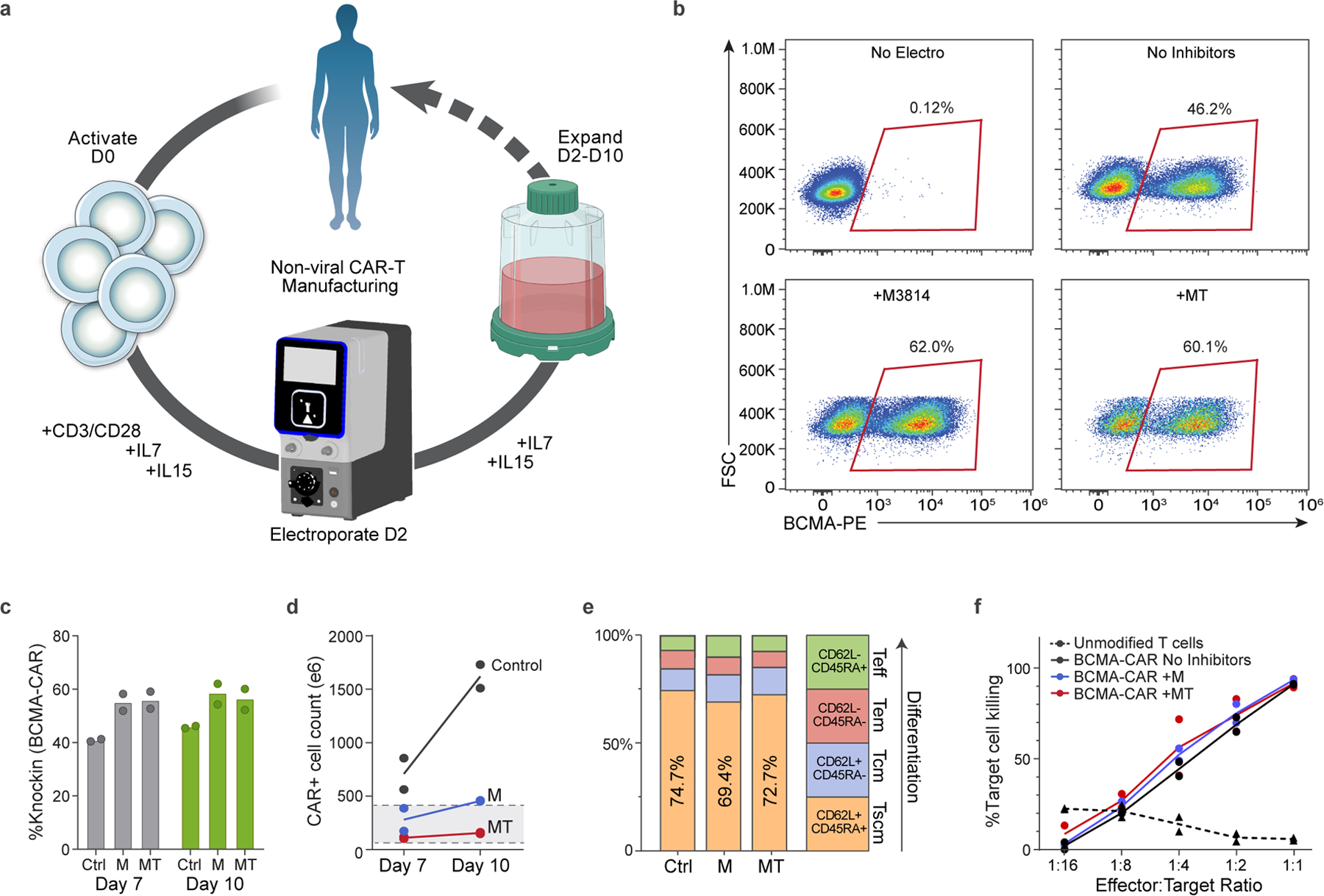
a, Diagram of nonviral CAR-T cell manufacturing process. T cells are isolated from peripheral blood and activated on day 0 with anti-CD3/anti-CD28 Dynabeads, IL-7 and IL-15. Cells are electroporated using the Maxcyte GTx electroporator on day 2 with Cas9 RNPs+ssCTS HDRTs and then expanded for a total of 7–10 days using G-Rex 100M culture vessels supplemented with IL-7+IL-15. b, Representative day 10 flow plots showing BCMA-CAR knock-in for control (no inhibitors), M3814 and MT conditions. c, BCMA-CAR knock-in rates on days 7 and 10 for each condition. d, Absolute number of CAR− cells on days 7 and 10. Gray box highlights anticipated patient doses of 50–400 × 106 CAR−T cells. e, T cell immunophenotypes on day 10 based on CD45RA and CD62L expression. f, In vitro killing of BCMA+MM1S multiple myeloma cell lines in comparison to unmodified T cells from same blood donors. Experiments performed with T cells from two independent healthy human blood donors represented by individual dots + mean (c,d,f) or mean alone (e); a was generated in part using graphics created by Biorender.com. M, M3814; Tscm, T stem cell memory; Tcm, T central memory; Tm, T effector memory; Teff, T effector.
