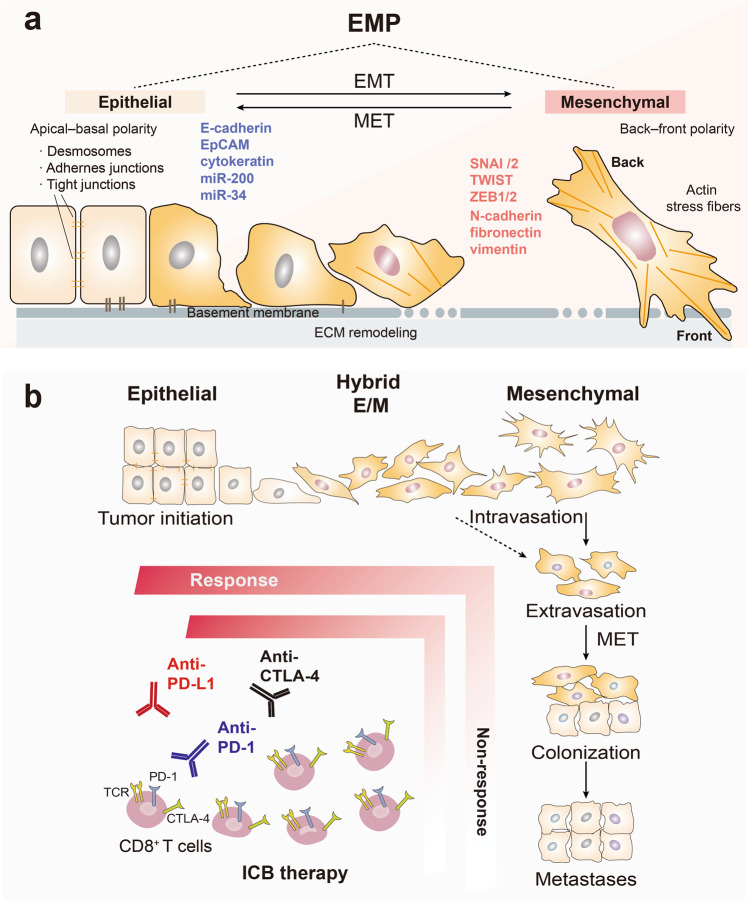
Fig. 1.
The relevance of EMP states and ICB response. a EMP refers to a broad spectrum of intermediate epithelial/mesenchymal (hybrid or partial E/M) phenotypic states between fully epithelial and fully mesenchymal of tumors with an active EMT or MET dynamic program. EMT is induced by a set of EMT-inducing transcription factors (EMT-TFs), like SNAI1/2, TWIST and ZEB1/2, that trigger epithelial cancer cells to undergo a series of molecular and morphological changes including tight junction dissolution, cell polarity alterations, cytoskeletal rearrangements, the loss of epithelial cell markers (e.g., E-cadherin, integrins, EpCAM, Claudin), take on appearance of mesenchymal cell phenotype, acquire increased motility, migration and apoptosis resistance, demonstrate elevated production of ECM components and emergence of mesenchymal cell markers (e.g., N-cadherin, vimentin, fibronectin). In contrast, mesenchymal cells can reverse to epithelial cells through activating a MET program. microRNAs such as miR-200 and miR-34 families are crucial for this process, which are regulated in double-negative feedback loops with the EMT-TFs ZEB1/2 and SNAI1/2, respectively. b Cancer cells exhibit different EMP states during cancer progression from primary tumors to distant metastases. Benign cells are initially transformed into cancerous epithelial cells. Cancer cells undergo dynamic phenotypic changes that are characterized by an epithelial state, an intermediate hybrid E/M hybrid state and a mesenchymal state through EMT or MET. Cancer cell EMP is a critical determinant in regulating immune escape and resistance to ICB therapies, e.g., anti-PD-1, anti- PD-L1 and anti-CTL-4. CTL-4 cytotoxic T-lymphocyte-associated protein 4, E/M epithelial/mesenchymal, EMT epithelial to mesenchymal transition, EMP epithelial mesenchymal plasticity, ICB immune checkpoint blockade, MET mesenchymal to epithelial transition, MiR micro RNA, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1