Highlights
-
•
GD2 is a promising target in CAR-T therapy for GBM.
-
•
GD2 CAR-T in combination with Nivolumab exhibit effective anti-GBM activities in vitro and in vivo.
-
•
The optimal therapeutic outcome is obtained using the medium dose of GD2 CAR-T with Nivolumab, which displayed the highest effectiveness.
Keywords: Glioblastoma, GD2, CAR-T, PD-1, Tumor microenvironment
Abstract
Glioblastoma (GBM) is a common primary brain tumor with poor clinical prognosis. Although CAR-T therapy has been trialed for treatment of GBM, the outcomes are sub-optimal possibly due to exhaustion of T cells and life-threatening neurotoxicity. To address these issues, a combined therapeutic strategy was tested in the current study using GD2 CAR-T together with Nivolumab - an anti-PD-1 monoclonal antibody. An effector-to-target co-culture system was established to evaluate the short-term and long-term cytotoxicity of CAR-T, as well as to investigate the inhibitory activity and T cell exhaustion associated with the PD-1/PD-L1 signaling pathway. Orthotopic NOD/SCID GBM animal models were generated to evaluate the safety and efficacy of the combined therapeutic strategy at various dosages of GD2 CAR-T with Nivolumab. GD2 CAR-T exhibited significant antigen-specific cytotoxicity in a dose-dependent manner in vitro. The persistence of cytotoxicity of GD2 CAR-T could be enhanced by addition of Nivolumab in the co-culture system. Animal studies suggested that GD2 CAR-T effectively infiltrated into tumor tissue and significantly hampered tumor progression. The optimal therapeutic outcome was obtained via using the medium dosage of CAR-T with Nivolumab, which displayed the highest efficacy in extending the survival up to 60 days. Further investigation of toxicity revealed that high-dosage of GD2 CAR-T could induce tumor apoptosis through p53/caspase-3/PARP signaling pathway. This study suggests that GD2 CAR-T in combination with Nivolumab may offer an improved therapeutic strategy for treatment of GBM.
Introduction
Glioblastoma (GBM) is a highly aggressive and undifferentiated glioma that frequently occurs in adults aged between 21 and 50 years old. The average incidence rate of GBM is about 3.19 individuals per 100,000 population [1]. Patients with GBM always have a median survival of < 15 months upon diagnosis [2]. Maximum surgical resection following radiotherapy and/or chemotherapy using temozolomide (TMZ) has become the standard therapy for treatment of newly diagnosed GBM patients [3]. However, recurrence is inevitable following a median period of 32–36 weeks [4]. Numerous long non-coding RNAs (lncRNAs) are deregulated or differentially expressed in GBM, and possess unique regulatory functions in GBM cells, ranging from high invasion/migration to recurrence [5]. Additionally, c-Myc is an oncogene signaling pathway capable of regulation of biological processes such as apoptotic cell death, proliferation, survival, differentiation, triggering much interest in the field with its role in different cancers, particularly brain tumors [6]. Thus, a novel effective therapeutic approach is urgently required for the treatment of GBM. Chimeric antigen receptor T cells (CAR-T) are genetically modified T cells that can be activated upon recognition of tumor-associated antigens (TAAs) through the single-chain fragment variable (scFv) region of the CAR molecule, resulting in highly efficient and specific tumor eradication via an MHC-I-independent pathway. In this study, we investigated the anti-glioblastoma effects of a combinatory approach using Nivolumab (a PD-1 antibody) and a second-generation GD2-targeting CAR-modified T cells.
GD2, an oncofetal differentiation antigen, is highly expressed on several solid tumors but rarely on normal tissues [7,8]. Recently, several preclinical and clinical studies have suggested that GD2, a specific target for GBM treatment, exhibits encouraging anti-tumor effects using the CAR-T-based immunotherapeutic strategy [8,9]. However, several obstacles, including the heterogeneity of the tumor antigen, loss of antigens, and hostile tumor microenvironment (TME), need to be overcome to obtain better anti-tumor effects [10]. Immune checkpoint inhibitor therapy can reverse the exhaustion of CAR-T in elapsing patients by shaping the TME. Moreover, blockade of the inhibitory receptor of T cells prevents the exhaustion induced by binding of the receptor to its cognitive ligand, such as PD-1 and PD-L1 [11]. In addition to immunosuppression, a barrier to the widespread use of CAR-T therapy for solid tumors is non-specific toxicities, including cytokine release syndrome (CRS) and neurotoxicity. IL-6 is an important indicator of the severity of CRS following CAR-T treatment. Moreover, IFN-γ could upregulate PD-L1 expression on glioblastoma cells in the GBM microenvironment, leading to CAR-T exhaustion via the PD-1/PD-L1 signaling pathway [12,13]. Herein, improvement of the safety and efficacy has been a pressing need in the treatment of solid tumors, including GBM.
To overcome these limitations, a combinatory therapeutic strategy was developed in the current study by employing GD2-specific CAR-T together with anti-PD-1 monoclonal antibody, Nivolumab, to verify whether the immune checkpoint inhibitors (ICI) could 1) enhance the anti-tumor activity mediated by CAR-T to reduce the infusion dose, and 2) ameliorate the TME-based inhibitory effect to enhance the anti-tumor activity of CAR-T in vitro and in vivo.
Material and methods
Cell lines and cultivation
Human glioblastoma cell lines, including T98G, U87, U251, and A172, were purchased from Procell Company (Wuhan, China) and cultured according to the manufacturer's instructions. T98G and U87 were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco, USA) containing 10% fetal bovine serum (FBS) (Gibco, USA). U251 and A172 were cultured in Minimum Essential Medium (MEM) (Gibco, USA) with 10% FBS. The patient-derived cell line GBM4 was established in our lab using tumor tissue from a patient diagnosed with high-grade glioma and maintained in DMEM medium supplemented with 10% FBS, the isolation of the primary cell line GBM4 was approved by Ethical and Institutional Review Board of Xuanwu Hospital Capital Medical University. HEK293T was kindly gifted by the Palmer lab at Stanford University and maintained in DMEM medium supplemented with 10% FBS. All cell lines were maintained in a humidified incubator with 5% CO2 at 37 °C.
CAR construction, lentivirus production, and T-cell transduction
Anti-GD2 CAR consisted of four components, 1) the extracellular antigen-recognizing single-chain variable fragment (scFv) targeting GD2 was derived from Fab fragment of the monoclonal antibody 3F8 [14], 2) the CD8α-derived hinge and transmembrane region, 3) the co-stimulatory domain from 4-1BB, and 4) the CD3ζ intracellular signaling domain. The GFP reporter was present in the C-terminal via P2A. All these components were cloned into a third-generation lentiviral plasmid backbone as previously described [15]. For lentivirus production, the HEK293T cell line was used for the GD2-specific CAR-encoding lentivirus package. Briefly, the GD2-specific CAR-encoding plasmids pLP1, pLP2, and pVSV-G (Addgene, USA) were co-incubated with Lipofectamine 3000 reagent (Invitrogen, USA) for 15 min and then added to the HEK293T supernatant for transient transfection. After 48∼72 h, the supernatant was ultra-centrifuged at 20,000 × g for 2 h. The virus pellet was resuspended in X-VIVO15 (Lonza, USA) and stored at –80 °C.
Commercially available human PBMCs (Lonza, Switzerland) were isolated by gradient centrifugation using the Ficoll-Paque technique (Cytiva, USA). CD3+ T cells were purified and activated by CD3/CD28 magnetic beads (Gibco, USA), as described previously. After culturing for 24∼48 h, GD2-specific CAR-encoding lentivirus with multiplicity of infection (MOI) of 50 was added to the culture supernatant for transfection supplemented with 10 µg/mL polybrene (Santa Cruz, USA). The medium was replaced with fresh culture medium after 12 h. GD2-specific CAR-T was stained with biotinylated protein L and streptavidin-coupled PE (BD, USA). The expression level of CAR was tested and analyzed by flow cytometry assay.
CAR-T-mediated cytotoxicity assay
Glioblastoma cells (1 × 105 cells per well) were co-cultured with GD2-specific CAR-T or GFP T cells in various effector-to-target (E/T) ratios ranging from 0.5:1 to 8:1 for 5 h. The supernatants were harvested for detection using the Non-Radioactive Cytotoxicity kit (Promega, USA), as described previously [15].
Cytokine secretion assay
Cytokines secreted from supernatants from GFP T cells or GD2-specific CAR-T co-cultured with glioblastoma cell lines were evaluated using ELISA. After co-culture for 5 h, the supernatants (from 8:1 and 4:1 E/T ratios) were harvested, and the levels of IFN-γ and TNF-α were measured using an ELISA kit (NeoBioscience) as described previously [15].
Flow cytometric assay
Glioblastoma cell lines were incubated with PE-labeled mouse-anti-human GD2 and mouse-anti-human PD-L1 antibodies (Biolegend, USA) or control antibodies of related isotypes (Biolegend, USA) for 30 min at room temperature. GD2-specific CAR-T were collected on day 7 and day 14 and then stained for the population assay with primary antibodies against the following anti-human antibodies: CD3-PE, CD4-APC, CD8-PerCP, PD-1-PE, PD-L1-APC, CD45RO-PE, and CD197-APC (Biolegend, USA) according to the manufacturer's instructions. Flow cytometry was performed using FACS Calibur (BD, USA). All data were analyzed using the FlowJo V10.
In vivo orthotopic glioblastoma models
Female NOD.Cg-PrkdcscidIl2rgtm1Vst/Vst (NPG) mice aged 6–8 weeks old were purchased from Beijing Vital-River Animal Technology Company. The U87 cell line with a constant expression of luciferase (U87-Luci) was purchased from Beijing View-solid Biotech company and cultured according to the manufacturer's instructions (DMEM containing 10% FBS). To establish the GBM animal model, 1 × 105 U87-Luci cells were orthotopically injected into the ventral posterior medial nucleus (VPM) of mouse thalamic nuclei (coordinates with bregma: ML-1.5 mm, AP-2 mm, DV-3 to −4 mm). After 6 days, all mice were intraperitoneally injected with 200 mg/kg cyclophosphamide (Baxter, USA) to deplete the host lymphocyte compartments. After 24 h, 2 × 106 GFP T cells or GD2-specific CAR-T at various dosages (5 × 105, 1 × 106, 2 × 106) were injected into mice through the tail vein injection. Mice were given an intraperitoneal injection of 10 mg/kg Nivolumab (Selleck, China) following CAR-T infusion. The GBM tumor burden was monitored using the IVIS Lumina K Serial III System (Caliper, USA) after 10 min since luciferin (Vital-River, China) injection. Mice were imaged once weekly after CAR-T injection and would be euthanized if they met the criteria for euthanasia (neurological deficits, 20–30% weight loss, signs of distress). The animal experiment was approved by the local institutional review board.
Immunofluorescence and immunohistochemical staining
Animal brain tissues were collected and fixed in 4% PFA. Before the immunofluorescence (IF) and immunohistochemistry (IHC) assay, brains were submerged in 30% sucrose buffer for 2–3 days. Briefly, brain sections were cut into 20 µm slides and blocked with 5% donkey serum (Sigma-Aldrich, USA) for 30 min at room temperature. Then, the slides were incubated with primary antibodies against several proteins, including Ki67 (Abcam, USA), IL-6 (Cell Signaling Technology, USA), GD2 (Santa Cruz, USA), IFN-γ (Abcam, USA), TNF-α (Abcam, USA), PD-1 (Cell Signaling Technology, USA), NeuN (Cell Signaling Technology, USA), Iba1 (Abcam, USA), p53 (Abclonal, China), active caspase-3 (Beyotime Biotechnology, China), and cleaved-PARP (Abclonal, China), at a dilution of 1:200, at 4 °C overnight. For IF staining, mouse brain slides were washed thrice with PBS buffer (Gibco, USA) and incubated with related secondary antibodies conjugated with FITC, Cyanine3, or Cyanine5 (ThermoFisher, USA). For IHC staining, the reaction was detected using DAB IHC Detection Kit (Elabscience, USA), while diaminobenzidine (DAB) was used as a chromogen. Slides were counterstained with hematoxylin and washed thrice with PBS buffer. Images of IF were detected and captured by confocal scanning (Leica, Germany), while images of IHC were observed and captured by the Case-Viewer software (Hungary). Quantitative analyses of double-positive cells in IF staining were performed by ROI Manager of ImageJ software according to the publisher's instructions (https://imagej.nih.gov/ij/docs/menus/analyze.html#manager), and quantitative analyses of IHC staining were performed by IHC Tool Box of ImageJ software according to instructions (https://imagej.nih.gov/ij/plugins/ihc-toolbox/index.html).
Statistical analysis
All data are presented as mean ±SEM. Two independent groups were analyzed using Student's t-test. Multiple groups were statistically compared using two-way repeated-measures ANOVA. P-values of ≤ 0.05 were considered statistically significant. All data were analyzed using the statistical software GraphPad Prism v8.0 (USA).
Results
Generation and population assay of GD2-specific CAR-T cells
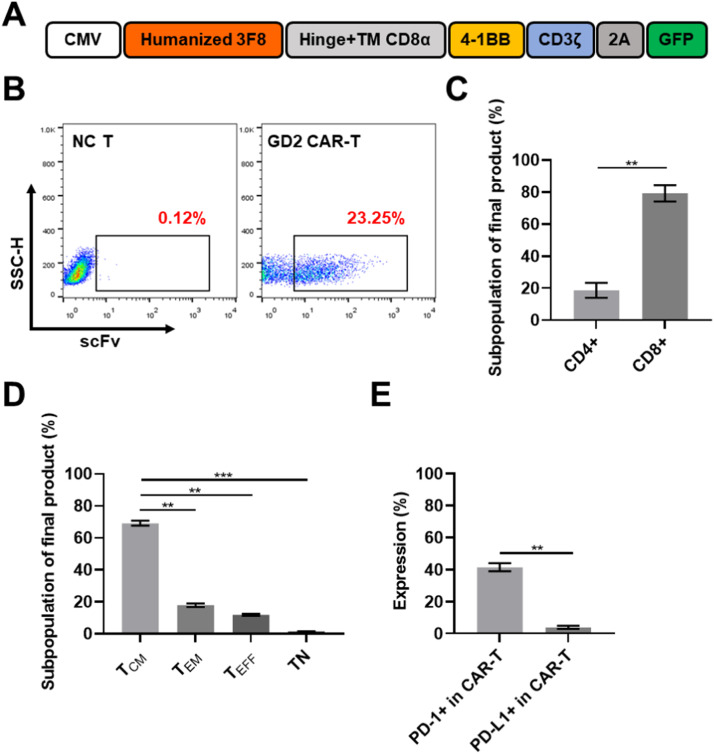
The second generation GD2-specific CAR, containing a 4–1BB co-stimulation domain and a CD3ζ stimulatory domain, was constructed as previously described [15]. Briefly, the GD2-targeting scFv sequence derived from the mouse anti-human GD2 monoclonal antibody 3F8 was commercially synthesized [14]. The above-mentioned sequence was then subcloned into the lentivirus expression plasmid, pLenti6.4, encoding CD19-targeting CAR to replace the counterpart of the scFv region to generate full-length GD2-specific CAR. By removing the terminator of CAR, the GFP gene was fused with CAR on the P2A sequence at the 3′ terminal as a reporting molecule. The schematic diagram is presented in Fig. 1A and Supplementary Fig. 1A. After cultivation in vivo for 12–14 days, the transduction efficiency of CAR was evaluated by flow cytometry. Lentivirus encoding GFP was used as the mock control. The results indicated that the transgenic efficacy of GD2-specific CAR was 23.0 ± 2.5%, which was lesser than the GFP control (58.0 ± 4.5%) on day 12 (Fig. 1B, Supplementary Fig. 1G). Sub-population assay of the final products revealed that the percentage of CD8+ T cells was about 79.2 ± 5.1%, which was greater than the corresponding CD4+ subpopulation (18.55 ± 4.65%) (Fig. 1C). The results associated with functional sub-populations of the final product indicated that the proportion of TCM (central memory T cells) (CD45RO+, CD197+) was 69.15 ± 1.55%, TEM (effector memory T cells) (CD45RO+, CD197-) was 11.9 ± 0.6%, TEFF (T effector cells) (CD45RO-, CD197-) was 17.8 ± 1.1%, and TN (naïve T cells) (CD45RO-, CD197+) was 1.4 ± 0.5% (Fig. 1D). Moreover, it was observed that while the proportion of PD-1+ CAR-T was 42.25 ± 2.65% in total GD2 CAR-T, the sub-population of PD-L1+ was low (4.5 ± 0.8%) (Fig. 1E). These results exhibited that GD2-specific CAR fused with GFP was successfully transduced and expressed in the primary CD3+ T cells. The cultivation system established in this study could be effectively used to generate GD2-specific CAR-T while being more feasible in promoting the proliferation of central memory T cells (TCM) of CAR-T cells, and would enhance anti-tumor effect of CAR-T cells against solid tumors [16].
Fig. 1.
Construction of GD2-targeting CAR and characteristics of GD2 CAR-T cells. A. Schematic diagram of the construction of GD2-specific CAR. The scFv region was derived from the Fab fragment of the monoclonal antibody 3F8. B. Expression of GD2-specific CAR on transduced T cells on day 12 following transduction. C. Population assay of the final products after cultivation for 12 days. The proportion of the CD8+ subpopulation was remarkably greater than that of CD4+ in the CAR-T cells. D. Subpopulation of memory T cells in the final products. TCM (central memory T cells) was the dominant subpopulation in GD2 CAR-T compared to TEM (effector memory T cells), TEFF (T effector cells), and TN (naïve T cells). Subpopulations of different T cells were identified as follows: TCM, CD45RO+/CD197+; TEM, CD45RO+/CD197-; TEFF, CD45RO-/CD197-; TN, CD45RO-/CD197+. E. Expression of PD-1 and PD-L1 in GD2 CAR-T after cultivation for 12 days.
Cytotoxicity against glioblastoma cell lines mediated by GD2-specific CAR-T cells
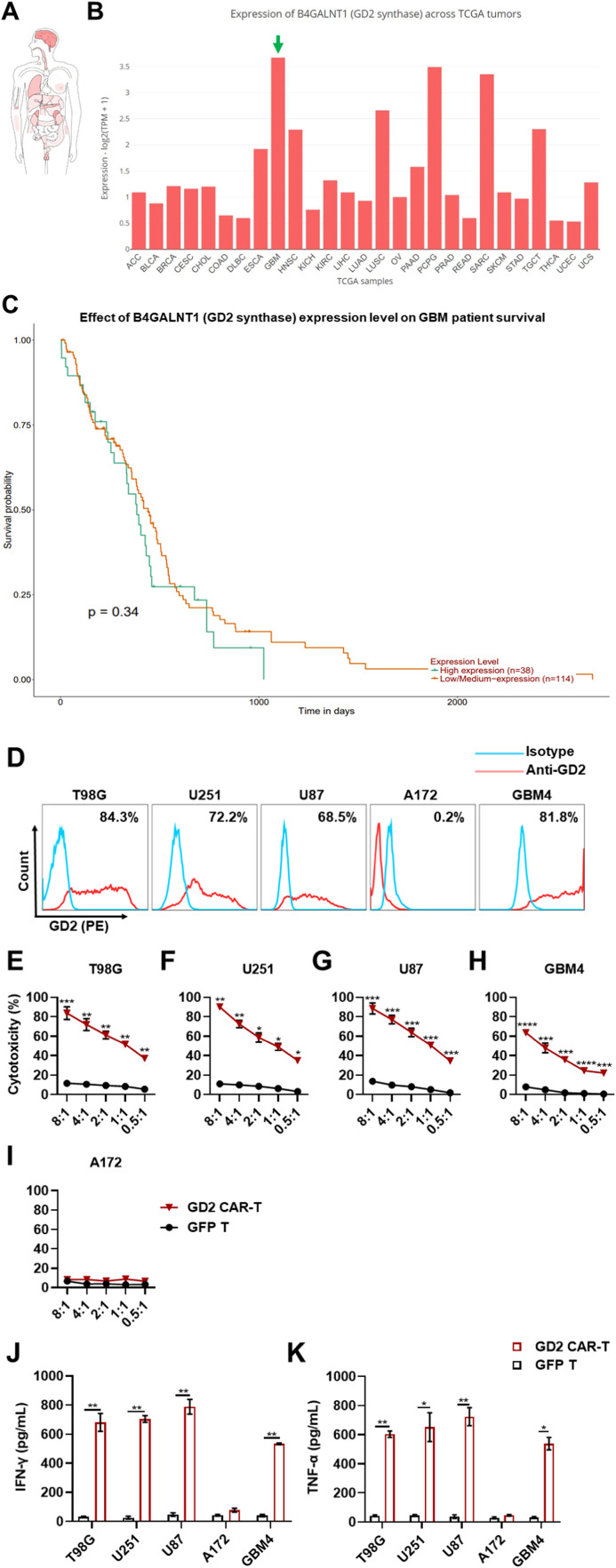
According to several previous reports, GBM displayed high inter-tumoral and intra-tumoral heterogeneity, indicating that GD2-specific CAR-T probably exhibited inconsistent anti-tumor efficacy against GBM cell lines with various levels of GD2 expression [17]. To verify our hypothesis, we first analyzed the publicly available online databases describing the expression of B4GALNT1 (GD2 synthase) in tumors compared to normal tissues. According to the GEPIA2 database (http://gepia2.cancer-pku.cn/#index), tumors derived from the brain, adrenal gland, lung, testis, and kidney displayed high levels of B4GALNT1 expression in the rank of top 5 (Fig. 2A). Pan-cancer analysis from UALCAN (http://ualcan.path.uab.edu/index.html) demonstrated that the expression of B4GALNT1 was markedly upregulated in GBM, and the probability of survival of GBM patients with high B4GALNT1 expression was much shorter than those with low or medium levels of expression (Figs. 2B, 2C). Then, various GBM cell lines, including T98G, U251, U87, A172, and a patient-derived cell line GBM4, with different expression levels of GD2, were used to detect the CAR-specific cytotoxicity by lactate dehydrogenase (LDH) assay. Flow cytometric assays suggest that the intensity of GD2 expression was 84.3%, 72.2%, 68.5%, 0.2%, and 81.8% in the above-mentioned cell lines, respectively (Fig. 2D). An in vitro co-culture system established by the effectors to target cells was used to evaluate the sensitivity and specificity of CAR-mediated cytotoxicity against GBM cell lines. Three GD2-targeting CAR constructions were generated (Supplementary Fig. 1A), namely, scFv of GD2–1, GD2–2 derived from 3F8 mAb [14], and scFv of GD2–3 derived from KM666 mAb [18] (Table 1). Consequently, cytotoxicity and cytokine secretion of the three GD2-targeting CAR-T cells against GBM cell lines were compared. It was observed that GD2–2 CAR-T exhibited more effective antigen-dependent cytotoxicity and cytokine secretion compared to GD2–1 and GD2–3 CAR-T (Supplementary Figs. 1B-1F and 1H-1I). Herein, GD2–2 CAR-T was used for evaluation in the subsequent studies and named GD2-specific CAR-T. Compared with GFP-transduced T cells, GD2-specific CAR-T exhibited significantly stronger cytotoxic activity against all GD2-positive GBM cell lines, including T98G, U251, U87, and GBM4, in a dose-dependent manner (Figs. 2E-2I). The range of cytotoxic efficacy varied from 12.1 ± 1.24% to 88.47 ± 9.61%, with the elevation of E/T ratio (from 0.5:1 to 8:1). However, the CAR-mediated anti-tumor effect was not significant in the cytotoxic assay against A172, a GD2-negative GBM cell line. To verify whether inflammatory cytokines were involved in the CAR-associated anti-tumor activity, IFN-γ and TNF-α, the two pivotal cytotoxic cytokines, were detected by ELISA. The concentrations of both IFN-γ and TNF-α were significantly elevated up to ∼700 pg/mL and ∼800 pg/mL, respectively, after 8:1 and 4:1 E/T incubation for 5 h (Figs. 2J and 2K). These values were significantly higher than counterparts from the GFP T cell group and after co-culture with the GD2-negative GBM cell line A172, respectively. Therefore, GD2-specific CAR-T showed significant anti-tumor activity against GBM cell lines expressing high and moderate levels of GD2 but not against GD2-negative GBM cell lines. IFN-γ and TNF-α were significantly increased during CAR-mediated tumor eradication in vitro, indicating that the designed CAR had high specificity to GD2-positive GBM cell lines, exerting effective cytotoxicity against tumor cells.
Fig. 2.
GD2 is a promising potential target for the treatment of GBM. A. The expression profile of B4GALNT1 (GD2 synthase) in different tumors. This gene was the most expressed in the brain tumor tissues among tumors derived from different tissues. B. The analysis of B4GALNT1 expression in different tumors according to samples from the TCGA database. C. Correlation between the expression of B4GALNT1 and the survival of GBM patients. D. GD2 expression in different GBM cells and patient-derived cell lines (GBM4). GD2 displayed a significant heterogeneous expression profile in various GBM cell lines. E-I. GD2-specific CAR-T exhibited local cytotoxicity against GD2-positive GBM cell lines. CAR-T-mediated cytotoxicity was measured by effector-to-target cell co-culture. CAR-T was co-cultured with different GBM cell lines at various indicated E/T ratios (ranging from 0.5:1 to 8:1) for 5 h. The efficacy of cytotoxicity was detected by LDH-releasing assay. J and K. Cytokines, including IFN-γ and TNF-α, were measured by ELISA during the CAR-T-mediated cytotoxicity assay. Following the co-culture of CAR-T with GBM cell lines, the supernatants were collected. Three independent assays were performed and data were presented as mean ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. “ns” indicates statistically insignificant results.
Table 1.
The sequences of the 3 CARs used in the current study.
|
GD2–1: scFv from human-mouse chimera 3F8 (Patent: CN103347894B) MALPVTALLLPLALLLHAARPSIVMTQTPKFLLVSAGDRVTITCKASQSVSNDVTWYQQKAGQSPKLLIYSASNRYSGVPDRFTGSGYGTAFTFTISTVQAEDLAVYFCQQDYSSFGGGTKLEIKGGGGSGGGGSKPLPEVTDEYGGGGSGGGGSQVQLKESGPGLVAPSQSLSITCTVSGFSVTNYGVHWVRQPPGKGLEWLGVIWAGGITNYNSAFMSRLSISKDNSKSQVFLKMNSLQIDDTAMYYCASRGGHYGYALDYWGQGTSVTVSSTTTPAPRPPTPAPTIASQPLSLRPEACRPAAGGAVHTRGLDFACDIYIWAPLAGTCGVLLLSLVITLYCKRGRKKLLYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCELRVKFSRSADAPAYKQGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDKMAEAYSEIGMKGERRRGKGHDGLYQGLSTATKDTYDALHMQALPPR |
|
GD2–2: scFv from humanized 3F8 (Patent: CN103347894B) MALPVTALLLPLALLLHAARPSIVMTQTPAFLLVSAGERVTITCRASQSVSNDVTWYQQKAGQAPRLLIYSASNRYTGIPARFSGSGYGTEFTFTISSVQSEDFAVYFCQQDYSSFGGGTKLEIKGGGGSGGGGSKPLPEVTDEYGGGGSGGGGSQVQLVESGPGLVQPGRSLRLTCAVSGFSVTNYGVHWVRQPPGKGLEWLGVIWAGGITNYNSAFMSRLTISKDNSKNTVYLQMNSLRAEDTAVYYCASRGGHYGYALDYWGQGTLVTVSSTTTPAPRPPTPAPTIASQPLSLRPEACRPAAGGAVHTRGLDFACDIYIWAPLAGTCGVLLLSLVITLYCKRGRKKLLYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCELRVKFSRSADAPAYKQGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDKMAEAYSEIGMKGERRRGKGHDGLYQGLSTATKDTYDALHMQALPPR |
|
GD2–3: scFv from huKM666 positive control (Patent: CN106536563A) MALPVTALLLPLALLLHAARPQVQLQESGPGLVKPSQTLSITCTVSGFSLASYNIHWVRQPPGKGLEWLGVIWAGGSTNYNSALMSRLTISKDNSKNQVFLKMSSLTAADTAVYYCAKRSDDYSWFAYWGQGTLVTVSSGGGGSKPLPEVTDEYGGGGSENQMTQSPSSLSASVGDRVTMTCRASSSVSSSYLHWYQQKSGKAPKVWIYSTSNLASGVPSRFSGSGSGTDYTLTISSLQPEDFATYYCQQYSGYPITFGQGTKVEIKRTTTPAPRPPTPAPTIASQPLSLRPEACRPAAGGAVHTRGLDFACDIYIWAPLAGTCGVLLLSLVITLYCKRGRKKLLYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCELRVKFSRSADAPAYKQGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDKMAEAYSEIGMKGERRRGKGHDGLYQGLSTATKDTYDALHMQALPPR |
Improvement of the anti-tumor persistence of GD2-specific CAR-T in combination with Nivolumab
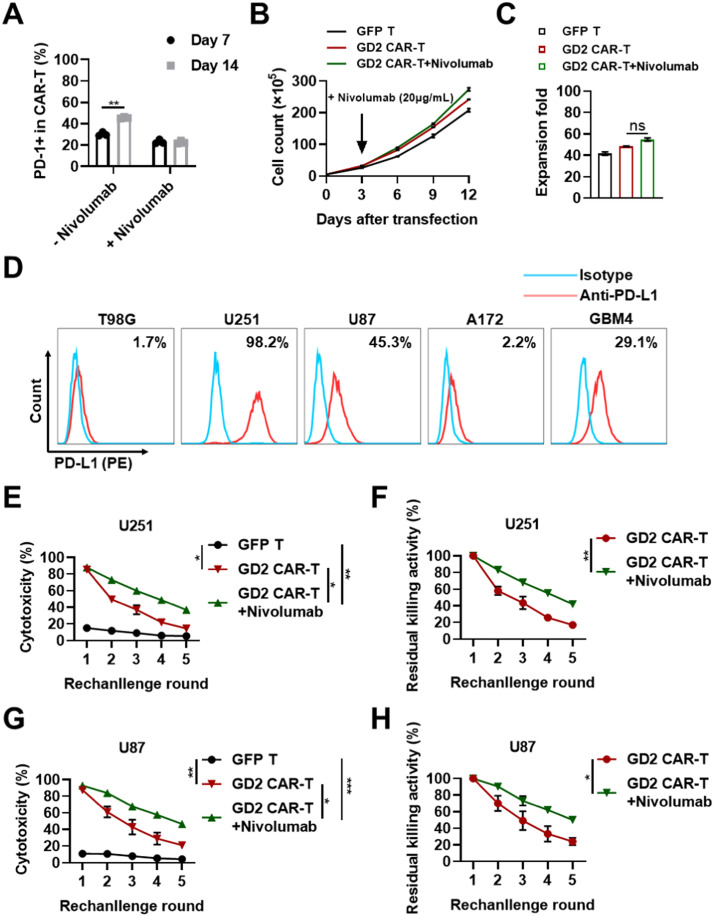
Although significant CAR-mediated anti-tumor activity was observed in vitro, the flow cytometry assay revealed that the proportion of PD-1+ GD2 CAR-T cells was increased from 30.23 ± 2.07% to 45.47 ± 0.83% from day-7 to day-14, indicating that the exhaustion of T cells could be enhanced during in vitro proliferation (Fig. 3A). It was also demonstrated that some GBM cell lines, including U251 (98.2%), U87 (45.3%), and GBM4 (29.1%), expressed high levels of PD-L1 (Fig. 3D). These findings suggested that the long-term cytotoxic activity of GD2-specific CAR-T was probably inhibited via the PD-1/PD-L1 pathway during anti-tumor action. Immune checkpoint inhibitors targeting PD-1 or PD-L1 have previously substantially improved the outcomes of patients with diverse cancers, although only 20–40% of the patients have benefitted from these new therapies [19]. Herein, we assumed that the anti-PD-1 antibody could inhibit the PD-1 expression of CAR-T cells during cultivation, as well as enhance the anti-tumor activity of GD2-specific CAR-T cells.
Fig. 3.
Nivolumab enhances the long-term cytotoxicity against GD2+/PD-L1+ GBM cell lines mediated by GD2-specific CAR-T cells. A. Nivolumab treatment inhibited the increase in PD-1 expression of T cells. PD-1 expression of GD2 CAR-T treated with or without Nivolumab (20 µg/mL) was determined by flow cytometry on day 7 and day 14. B. Proliferation profile of GD2 CAR-T cells with or without Nivolumab in vitro. Primary CD3+ T cells were purified and activated by Dynabeads conjugated with CD3/CD28 antibody. Lentivirus expressing GD2-specific CAR or GFP was added after activation for 24 h. Cells in the GD2 CAR-T + Nivolumab group were treated with Nivolumab at a final concentration of 20 µg/mL on day 3. Cells were counted at various indicated intervals and harvested on day 12. C. Expansion fold change of GFP T cells and GD2 CAR-T with or without Nivolumab in vitro. D. Expression of PD-L1 on different GBM cell lines and the patient-derived cell line GBM4, as measured by flow cytometry. E and G. Long-term cytotoxicity and relative fold change mediated by GD2-specific CAR-T with or without Nivolumab treatment. GD2-specific CAR-T were re-challenged with U251 and U87 for five rounds with an E/T ratio of 8:1. Supernatants were harvested after co-culture for 5 h for the LDH release assay. F and H. The relative fold change of cytotoxicity was calculated as the value of the first round as baseline divided by the values of the remaining rounds, respectively. Three independent assays were performed and the data were presented as mean ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001. “ns” indicates statistically insignificant results.
To test our hypothesis, the CAR-T culture was treated with the PD-1 monoclonal antibody Nivolumab (20 µg/mL) on day 3. The proliferation analysis indicated that the proliferation of GD2 CAR-T cells was not significantly increased following Nivolumab treatment. After cultivation for 14 days, GFP T cells and GD2 CAR-T cells increased by 41.5- and 48.5-fold, respectively, whereas the GD2 CAR-T cells increased by 54.8-fold following Nivolumab administration which was slightly higher than the other groups (Figs. 3B and 3C). However, the increase in the level of PD-1 expression was significantly inhibited. The proportion of PD-1+ GD2 CAR-T cells was dramatically increased from 30.23% ±2.07% on day 7 to 45.47 ± 0.83% on day 14 in the control group (Fig. 3A), whereas the corresponding values in the treatment group (with 20 µg/mL Nivolumab) were 23.1 ± 2.3% and 22.6 ± 1.5%, respectively. This suggested that Nivolumab could remarkably inhibit the increase of PD-1+ CAR-T sub-population to prevent the exhaustion of T cells during cultivation in vitro.
Furthermore, we studied the long-term cytotoxic effect of GD2 CAR-T in combination with Nivolumab against PD-L1-positive U251 and U87 in vitro. We observed that GD2 CAR-T cells, in combination with Nivolumab (20 µg/mL), exhibited more potent cytotoxic functions against U251 and U87 in the five-round repetitive challenges (Figs. 3E and 3G). In the case of the U251 cell line, GD2-specific CAR-T showed a cytotoxic efficiency of 23.4 ± 2.18% and maintained a residual elimination ability of 26.63 ± 2.35% in combination with Nivolumab at the fifth round of re-challenging (Figs. 3E and 3F), whereas, treatment with GD2-specific CAR-T alone showed cytotoxic efficiency of only 14.46 ± 0.98% and residual elimination ability of only 16.98 ± 1.56%. In the case of the U87 cell line, GD2-specific CAR-T in combination with Nivolumab demonstrated a cytotoxic efficiency of 40.47 ± 8.3% and residual elimination ability of 43.44 ± 7.83% at the fifth-round re-challenge, whereas treatment with CAR-T cells alone showed values of only 20.97 ± 3.38% and 24.08 ± 4.44%, respectively (Figs. 3G and 3H). These results indicated that the anti-tumor cytotoxicity of CAR-T cells was significantly exhausted without Nivolumab treatment. However, this exhaustion could be effectively inhibited by the PD-1 antibody, indicating that blockage of the PD-1 molecule could enhance the anti-tumor persistence of GD2-specific CAR-T. In summary, it was observed that treatment with Nivolumab greatly improved the persistent cytotoxicity of GD2-specific CAR-T in vitro.
GD2-specific CAR-T, in combination with Nivolumab, exhibits enhanced anti-tumor function in murine glioblastoma models
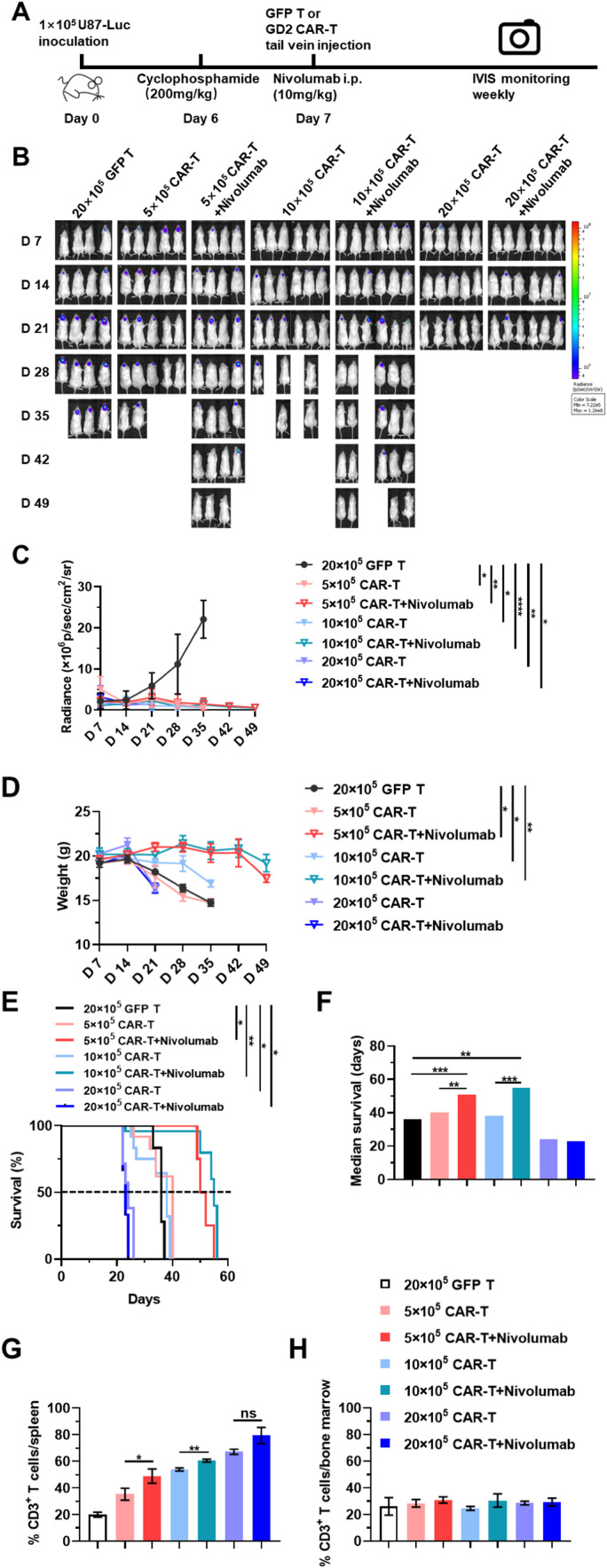
To evaluate the anti-tumor efficacy of GD2-specific CAR-T against GBM in vivo, animal models with orthotopic glioblastoma cells injection were established using NOD-SCID mice by implanting the U87-Luci cell line, as described in previous reports [9,20]. U87-Luci cells (1 × 105) were orthotopically injected into the ventral posterior medial nucleus (VPM) region of mouse thalamic nuclei (coordinates to bregma: ML-1.5 mm, AP-2 mm, DV-3 to −4 mm) (Fig. 4A). After six days, all mice received an intraperitoneal injection of cyclophosphamide (200 mg/kg) to deplete host lymphocytes and provide a moderate environment for the survival of re-injected T cells, as well as to eradicate Tregs in mice. On the following day, all mice were randomly divided into seven groups as indicated and then were administered the corresponding doses of GD2 CAR-T via tail-vein injection. GFP T cells (2 × 106 per mouse) were administrated in the mock group (Fig. 4A). IVIS imaging was performed weekly to evaluate the therapeutic effect of GD2-specific CAR-T with or without Nivolumab (10 mg/kg). Consecutive bioluminescence images indicated that all groups treated with GD2 CAR-T, particularly the 20 × 105 GD2 CAR-T group (with or without Nivolumab), showed a gradual decrease in the tumor burden, which was not observed in the mock group (Fig. 4B). The radiance of U87-Luci cells transplanted in all mice brains was significantly reduced following treatment with CAR-T (Fig. 4C, Supplementary Figs. 2A-2G). The increasing tumor burden might harm mice, as acute weight loss in mice treated with GFP T cells was recorded during the treatment period until the death of the animal (Fig. 4D). However, it was also observed that treatment with high-dose CAR-T (20 × 105/mice) could also exhibit a significant side-effect on mice, suggesting that mice treated with 20 × 105 GD2 CAR-T (with or without Nivolumab) had a shorter lifespan than the mock group. Notably, animals in the groups treated with 5 × 105 or 10 × 105 GD2 CAR-T with Nivolumab exhibited an extended median survival time of > 50 days compared to all other groups (Figs. 4E and 4F).
Fig. 4.
In vivo anti-tumor effects of GD2 CAR-T in combination with Nivolumab. A. Schematic outline of treatment with GD2 CAR-T with or without Nivolumab. NOD/SCID mice were intracranially injected with 1 × 105 U87-Luci cells. Six days later, xenografted mice were analyzed using IVIS Lumina. Mice showing a positive signal were randomly divided into seven groups and were pretreated with cyclophosphamide at a dose of 200 mg/kg. On the next day, mice received different indicated treatments and were monitored by total flux with radiance each week. B. Serial bioluminescence imaging of the progression and regression of tumors in each group at the indicated time points. C. Mean total flux values (photons/s) of each treatment group at the indicated time points. D. Mean weights in each treatment group at the indicated time points. E and F. Survival percentage and median survival time in each treatment group. G and H. Proportion of CD3+ T cells in the spleen and bone marrow of each treatment group. Tissue samples were collected from different treatment groups before mice were sacrificed. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001. “ns” indicates statistically insignificant results.
Then, to investigate the pharmacokinetic association between the dosage, treatment strategies, and therapeutic effect in vivo, the distribution of CAR-T in varying dosages was evaluated by flow cytometry assay. The percentage of CD3+ T cells was elevated proportionally with an increase in the infusion dosage, from 35.285% to 79.43%, which was significantly higher than the corresponding GFP T cells treatment group (19.97%). Moreover, a higher proportion of CD3+ T cells was detected in the combination treatment group compared to single treatment with the same infusion dosages, indicating that Nivolumab possibly promoted the homing of CAR-T to the spleen (Fig. 4G). The assay of bone marrow samples indicated that although there was no significant difference between the percentages of CD3+ T cells among different treatment groups, the percentages in the combined treatment group at each dosage level were slightly higher than those in the single-infusion groups. This suggested that Nivolumab could also enhance the bone marrow migration of GD2 CAR-T. A significant difference was observed in the 10 × 105 GD2 CAR-T groups in combination with Nivolumab, wherein the animals showed the best outcome following treatment (Fig. 4H). These results confirmed that medium or low dosages of GD2-specific CAR-T effectively eradicated GBM burden in vivo, especially in combination with Nivolumab. The 10 × 105 GD2 CAR-T, in combination with Nivolumab, exhibited the best anti-tumor function and optimally extended the survival of murine GBM models. This combination therapy provided a promising solution to improving the anti-tumor function of CAR-T in the treatment of GBM.
The potential mechanism associated with the toxicity of high-dose GD2 CAR-T in vivo
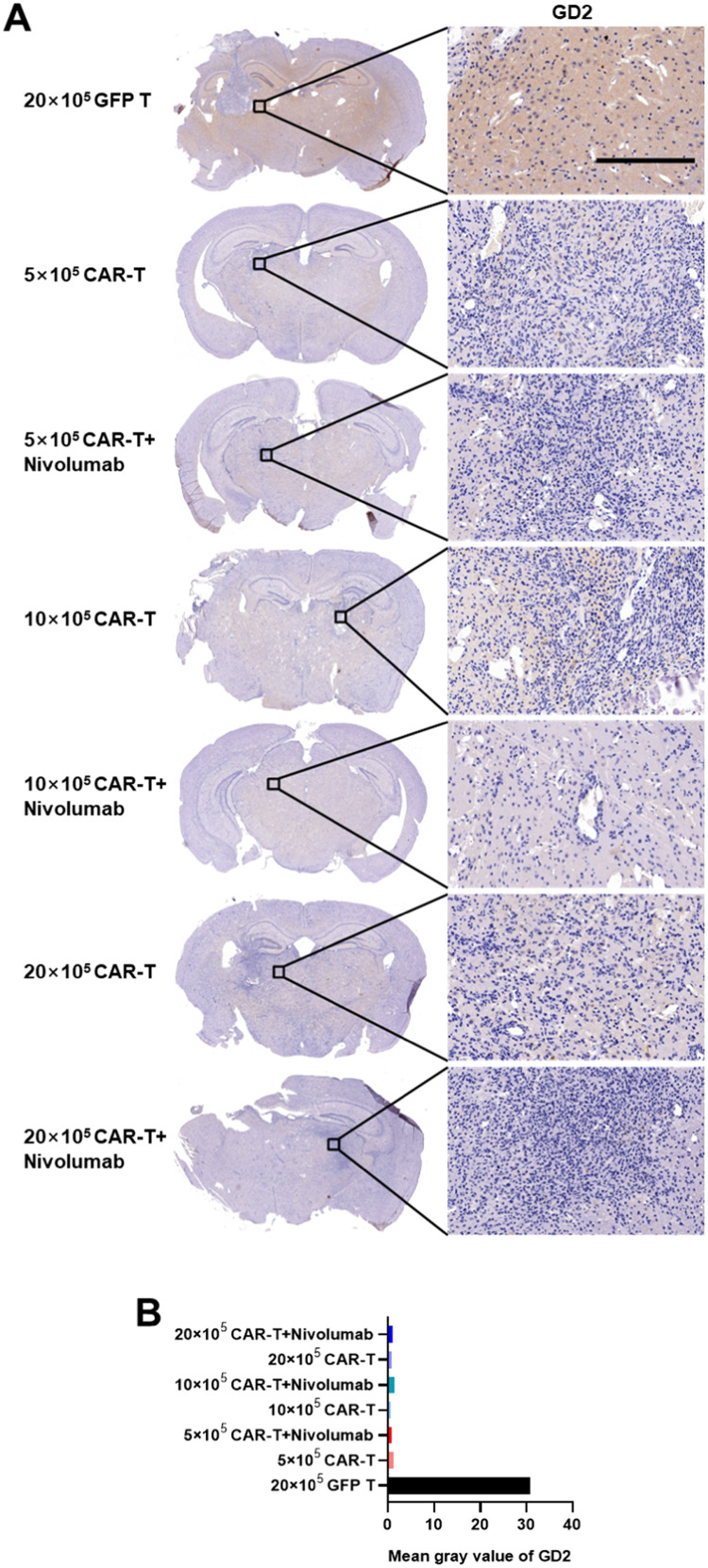
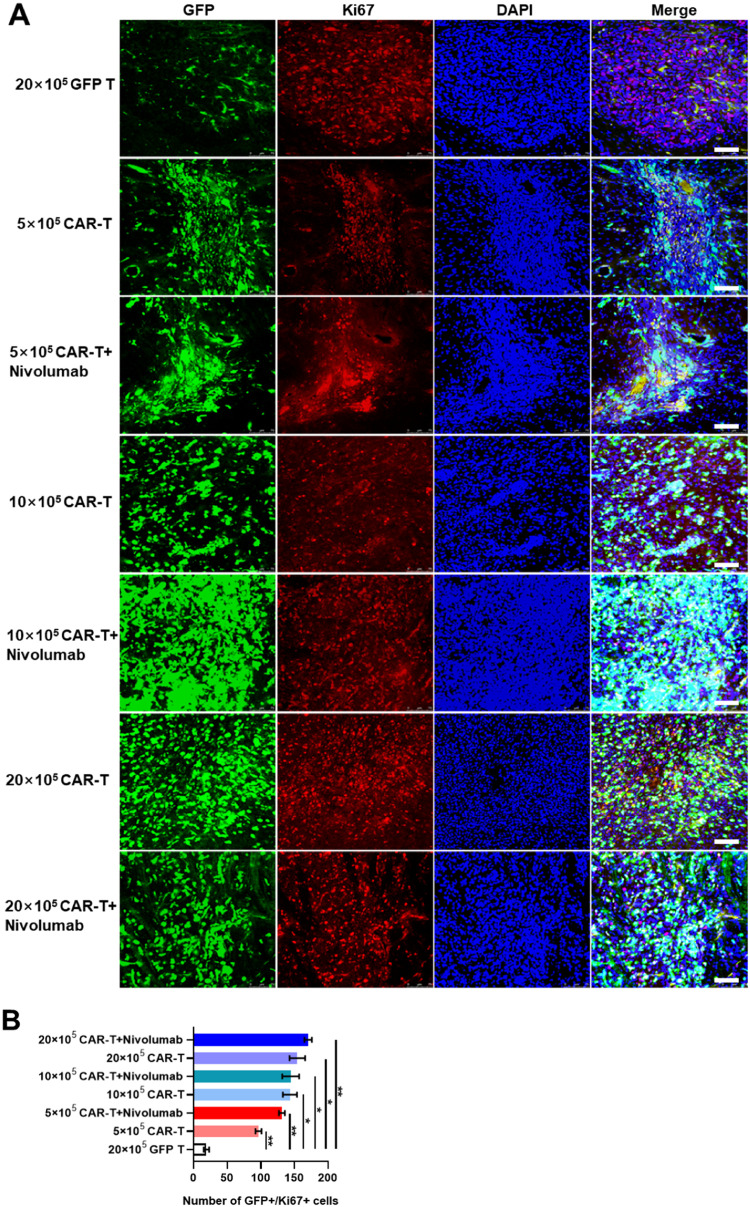
Various side effects, such as weight loss and shortened lifespan, were observed in different treatment groups. Thus, the infusion of CAR-T targeting the central neural system antigens probably induced several undesired threatening syndromes associated with anti-tumor inflammatory responses. To explore the possible mechanisms, various proteins and cytokines playing pivotal roles in the anti-tumor immune responses were analyzed in the brain tissues. First, through IHC analysis, it was observed that the GD2 expression level was significantly higher in the GFP T cells treatment group compared to those in the groups treated with CAR-T (Figs. 5A, 5B), suggesting that the tumor burden was probably effectively eradicated by GD2-specific CAR-T. Then, immunofluorescence assays were performed to determine the expression levels of proteins that are important in the apoptotic cascade and to explore the possible mechanism of shorter lifespan induced by high-dose CAR-T. The results of Ki67 staining revealed the presence of more GFP and Ki67 double-positive cells in each group treated with GD2-specific CAR-T than in the group treated with GFP T cells (Figs. 6A, 6B). This indicated that GD2-specific CAR-T could effectively proliferate and harbor significant persistent cytotoxicity. Moreover, whole brain scanning of mice treated with 20 × 105 GD2 CAR-T revealed more GFP and Ki67 double-positive signals in the left side of the brain, which was not observed in the group treated with GFP T cells. This demonstrated the better antigen-specific homing ability of GD2-specific CAR-T, as U87-Luci cells were implanted only in the left side of mouse brains and gradually migrated and infiltrate to the whole brain (Supplementary Fig. 3).
Fig. 5.
The expression of GD2 in the brains of GBM-bearing murine models established by the U87-Luci injection. A. GD2 was detected by immunohistochemical staining using a mouse-anti human GD2 monoclonal antibody. Nuclei were counterstained by hematoxylin. Scale bars represented 500 µm and 20 µm on the left and right panels, respectively. B. Means of gray density values of GD2 were measured using the ImageJ software according to the manufacturer's instructions.
Fig. 6.
The expression of Ki67 in brains of murine GBM models established using U87-Luci injection in different treatment groups. A. Immunofluorescent staining was used to detect Ki67 by mouse anti-human Ki67 monoclonal antibody and a secondary antibody conjugated with Cyanine3. GFP signal was represented as either GFP T or GD2-specific CAR-T cells. Nuclei were counterstained with DAPI. The scale bar was represented as 100 µm. B. Quantitative analyses of GFP+/Ki67+ cells in mouse brain slides as measured using ImageJ software.
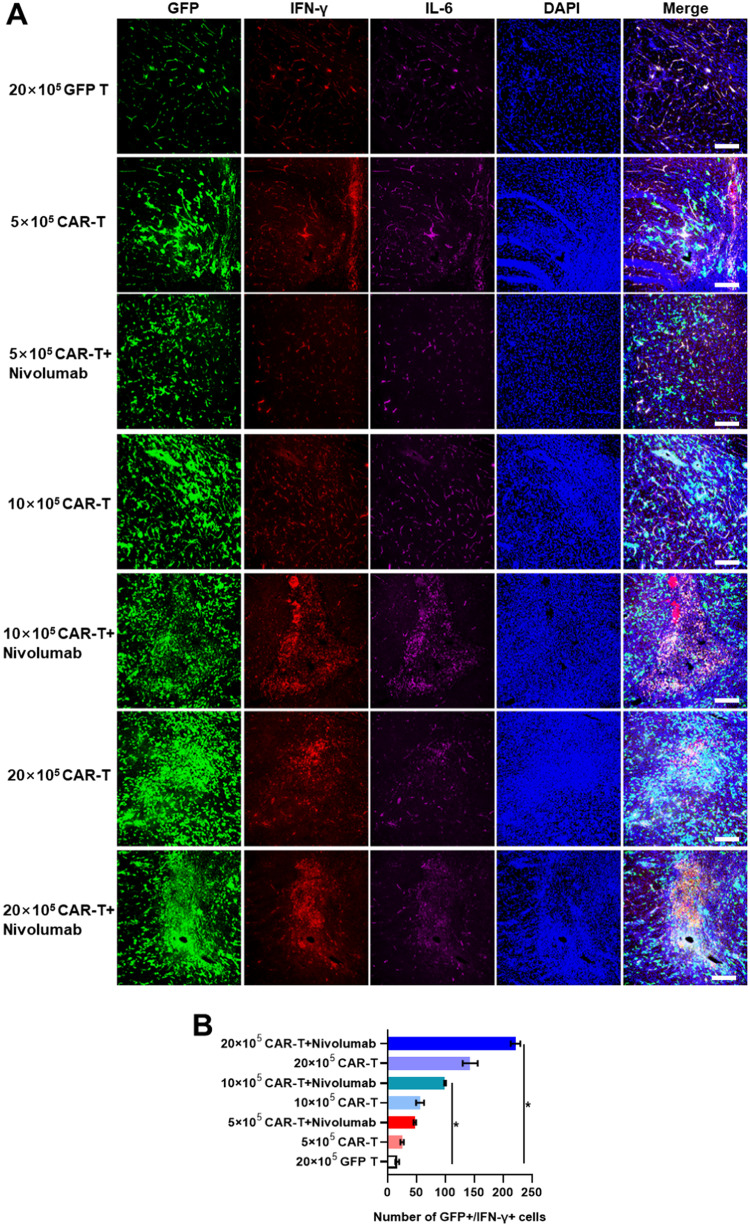
Moreover, it was observed that the PD-1 expression levels in groups treated with Nivolumab were lesser than those in the groups treated with GD2-specific CAR-T or GFP T cells alone, indicating that CAR-T with lower expression of PD-1 might exhibit a stronger and more potent cytotoxic response in the solid TME (Supplementary Figs. 4A and 4B). This result was corroborated by the longer survival of mice treated with 5 × 105 or 10 × 105 GD2-specific CAR-T in combination with Nivolumab. The level of pro-inflammatory cytokines agreed with the results of in vitro experiments, suggesting that treatment with a high dose of GD2 CAR-T resulted in higher expression of IFN-γ and TNF-α (Figs. 7A and 7B, Supplementary Figs. 6A and 6B). Besides, the expression of IL-6 in mouse brain was consistent in all groups, indicating that mice treated with 20 × 105 GD2 CAR-T might not die from CRS, as IL-6 is a major biomarker for monitoring CRS (Supplementary Figs. 5A and 5B, Supplementary Figs. 6A and 6B) [21,22]. Additionally, in contrast to GFP T treated group, IL6 level in mouse serum was consistent low in all GD2 CAR-T treated groups (Supplementary Fig. 10). Splenomegaly was not observed when mice were sacrificed, and there was no direct evidence for Homo sapiens GD2 expression in murine.
Fig. 7.
The expression of IFN-γ and IL-6 in the brains of murine GBM models established using U87-Luci injection in different treatment groups. A. IFN-γ and IL-6 were detected by mouse anti-human IFN-γ and rabbit anti-human IL-6, respectively. Secondary antibodies conjugated with Cyanine3 and Cyanine5 were used to stain IFN-γ and IL-6, respectively. Nuclei were counterstained with DAPI. The scale bar was represented as 100 µm. B. Quantitative analyses of GFP+/IFN-γ+ cells in mouse brain slides as measured using ImageJ software.
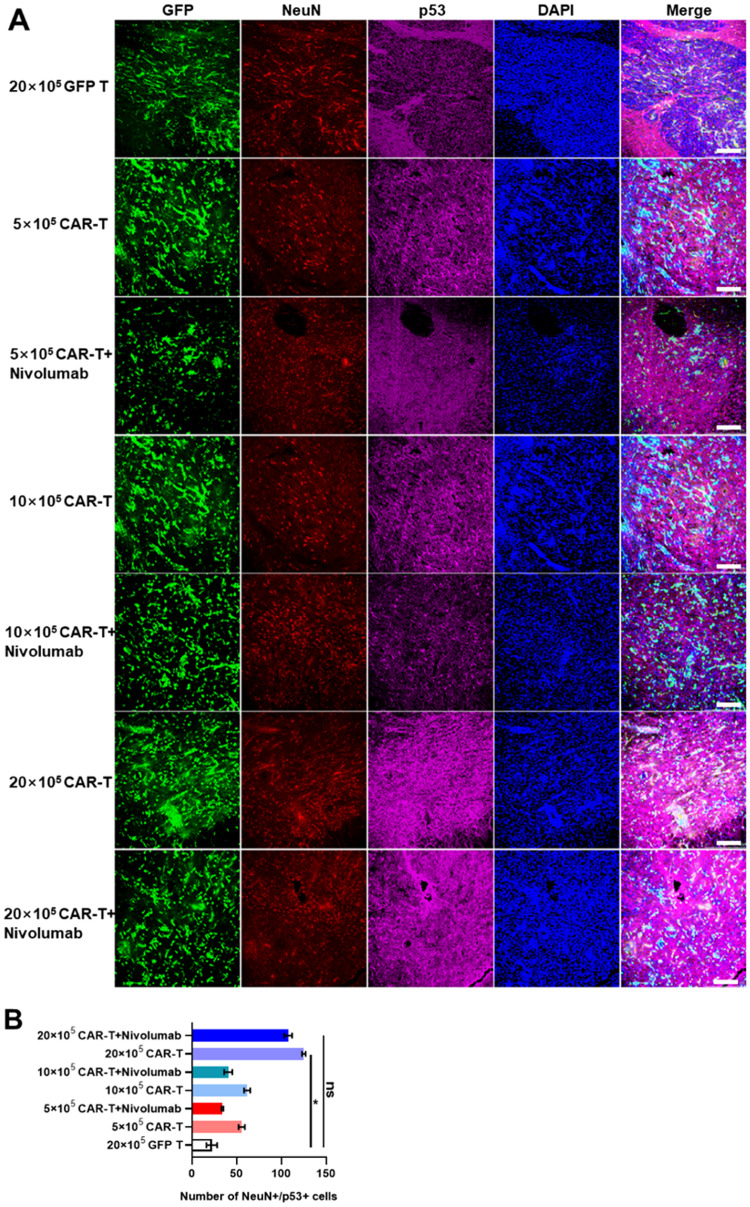
Furthermore, we investigated the reasons for the significantly shorter lifespan in the group treated with 20 × 105 CAR-T cells. The expression levels of several proteins vital to the apoptotic cascade were determined to explore their possible relationship with the lifespan of mice. It was observed that the expression of p53 and active caspase-3 was significantly increased, which coincided with the increasing level of PARP cleavage in groups treated with high-dose GD2 CAR-T (Figs. 8A and 8B, Supplementary Figs. 7A and 7B, Supplementary Figs. 8A and 8B, Supplementary Figs. 9A and 9B). These results indicated that 20 × 105 GD2-specific CAR-T might result in side effects in the mouse brain, including extensive apoptosis of normal brain cells, as most of the pro-apoptotic protein expression signals are co-localized in NeuN and Iba1 in mouse brain cells. This suggested that GD2-specific CAR-T-mediated pro-inflammatory cytokines may induce the apoptosis of neurons or microglia through the p53-caspase-3-PARP signaling pathway, causing neurotoxicity, which led to the significant shortening of the lifespan during treatment. Besides, when 20 × 105 GD2-specific CAR-T treated mice were close to death, severe weight loss was indeed observed, and mice were feeding and physically inactive.
Fig. 8.
The expression of NeuN and p53 in the brains of murine GBM models established using U87-Luci injection in different treatment groups. A. NeuN and p53 were detected by goat anti-mouse NeuN and rabbit anti-human p53, respectively. Secondary antibodies conjugated with Cyanine3 and Cyanine5 were used to stain NeuN and p53, respectively. Nuclei were counterstained with DAPI. The scale bar was represented as 100 µm. B. Quantitative analyses of NeuN+/p53+ cells in mouse brain slides as measured using ImageJ software. *p ≤ 0.05, **p ≤ 0.01. “ns” indicates statistically insignificant results.
Discussion
In the current study, we first demonstrated that GD2 was indeed highly expressed in glioma tissues and some primary glioma cell lines. Then we designed the GD2-targeting CAR structures and generated the corresponding GD2 CAR-T cells. We detected a high proportion of CD8-positive T cells in GD2 CAR-T cells, suggesting that they might possess a strong cytotoxicity against glioma cells. And the proportion of central memory T cells was much higher compared with other sub-populations, which could be beneficial for T cell-mediated anti-tumor effects. Subsequently, we found that addition of Nivolumab could greatly decrease the surface PD-1 expression level on GD2 CAR-T cells and enhance the persistence of the cytotoxic ability of CAR-T cells against PD-L1-expressing U251 and U87 cell lines. Then GBM murine models were established, and the combination therapy could greatly reduce the tumor burden in the mouse brain, and no excessive IL-6 in brain or plasma was detected, indicating a high safety profile of GD2 CAR-T cells with or without Nivolumab. High doses of GD2 CAR-T cells might have caused a proapoptotic effect in the brain and shortened the survival of animals. Further study is warranted to investigate the relationship between doses of CAR-T cells and safety.
Disialoganglioside, widely known as GD2, is highly expressed on several solid tumors but rarely expressed on normal tissues. It plays a pivotal role in the initiation and development of tumors, including the proliferation, motility, migration, adhesion, and invasion of tumor cells. This feature made GD2 an ideal TAA and a promising target for cancer immunotherapy [23,24]. Chimeric antigen receptor T-cell (CAR-T) therapy has yielded great success in the treatment of B-cell malignancies. Recent results have also reported its benefits in CNS malignancies, including GBM. To date, IL-13Rα2, EGFRvIII, and HER-2 have been the most well-studied GBM-specific tumor surface antigens for CAR-T therapy, and some promising results have been reported. However, several safety issues still existed, and recurrence of tumor following CAR-T treatment remained an intractable problem.
Recently, a growing number of preclinical trials using GD2 CAR-T for treatment of GBM had been conducted, and the results were encouraging [25,26]. As demonstrated in Majzner's research, diffuse intrinsic pontine glioma (DIPG) and other H3K27M-mutated diffuse midline gliomas (DMG) are commonly fatal pediatric central nervous system tumors in which GD2 is highly expressed on H3K27M-mutant glioma cells. Three out of four patients receiving GD2 CAR-T treatment showed improved imaging and clinical performance, with toxic side effects mainly limited to the location of the tumor. These preliminary results indicated that GD2-specific CAR-T is a promising tool in the treatment of DIPG or DMG tumors with H3K27M mutations [20]. However, the hostile immunosuppressive environment, antigen escape, and physical barriers limit CAR-T-based strategies in the treatment of GBM. Among these challenges, the immunosuppressive environment plays a key role in the failure of CAR-T against GBM [25,27]. To solve this problem, Nivolumab, an IgG4 PD-1 immune checkpoint inhibitor antibody of human origin, was used in the current study to boost the persistence and survival of GD2-specific CAR-T and showed encouraging results.
Identification of novel tumor-specific antigens may be helpful in tackling the problems of antigen loss and antigenic heterogeneity in treatment of solid tumors. Another approach was to boost the non-specific anti-tumor effect by using immune checkpoint inhibitors, such as Nivolumab, which showed a promising adjunct effect in the current study [28], [29], [30]. Besides, considering the brain origin of GBM, the route of delivery for CAR-T administration would also be optimized to maximize its anti-tumor efficiency and persistence. In the treatment of blood cancers, early preclinical studies and clinical trials employed intravenous injection route for the delivery of CAR-T. Despite the promising results via systemic administration, local administration of CAR-T might be more effective in treating solid tumors [31], [32], [33]. Several studies have demonstrated that CAR-T cells could migrate into brain tumor lesions through the blood-brain barrier (BBB), exhibiting antigen-specific tumor killing, as also demonstrated in this study. A focused intra-cavity delivery might allow direct access of CAR-T to the tumor tissue and reduce CAR-T cell loss in the homing process towards tumor tissues, thus could lead to a more potent anti-tumor efficacy and less systemic toxicity compared to systemic administration. Moreover, as several tumor-associated antigens are also present on normal tissues, intra-cavitary delivery of CAR-T directly to the GBM site might reduce the “on-target off-tumor” cytotoxicity and potentially prevent some fatal side effects.
In conclusion, GD2-specific CAR-T, in combination with Nivolumab, could effectively kill GBM cells in an antigen-specific manner in vitro and eradicate tumor burdens and extend lifespan of murine glioblastoma models in vivo. This combinatory strategy offered an effective approach for treatment of solid tumors, including GBM, through applying effector CAR-T cells together with immune checkpoint inhibitors to remodel the tumor microenvironment which may lead to a more potent persistence and proliferation ability of CAR-T cells. However, an improved strategy targeting multiple TAAs, using an optimal delivery route and dosage needed to be contemplated in future studies to, hopefully, pave the road to clinical translation in GBM therapies.
Declarations
Ethics approval and consent to participate
The isolation of the primary cell line GBM4 was approved by Ethical and Institutional Review Board of Xuanwu Hospital Capital Medical University. Animal experiments were approved by Animal Care Committee of Xuanwu Hospital Capital Medical University.
Availability of data and material
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Funding
This work was supported by National Natural Science Foundation of China (81973351, 82171250 and 82173840). Beijing Talents Foundation (2017000021223TD03), Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five–year Plan (CIT & TCD20180333), Beijing Medical System High Level Talent Award (2015-3-063), Beijing Municipal Health Commission Fund (PXM2020_026283_000005), Beijing One Hundred, Thousand, and Ten Thousand Talents Fund (2018A03), and a postdoctoral fellowship for Guangji Zhang from Beijing Municipal Human Resources and Social Security Bureau (2021-ZZ-004).
CRediT authorship contribution statement
Guangji Zhang: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Validation. Yu Zhao: Project administration, Writing – review & editing, Validation. Zhongfeng Liu: Data curation, Formal analysis, Methodology, Validation. Weihua Liu: Data curation, Formal analysis, Validation. Huantong Wu: Data curation, Formal analysis, Validation. Xuan Wang: Data curation, Validation. Zhiguo Chen: Conceptualization, Formal analysis, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Doctor Zixuan Wang for providing help in data and figure processing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101663.
Appendix. Supplementary materials
References
- 1.Lippi G., Dechecchi M.C. Glioblastoma biomarkers: finding a needle in a haystack. J. Lab. Precis. Med. 2018;3:59. [Google Scholar]
- 2.Cheng W., Zhang C., Ren X., Wang Z., Liu X., Han S., Wu A. Treatment strategy and IDH status improve nomogram validity in newly diagnosed GBM patients. Neuro-oncology. 2017;19:736–738. doi: 10.1093/neuonc/nox012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis M.E. Glioblastoma: overview of disease and treatment. Clin. J. Oncol. Nurs. 2016;20:S2–S8. doi: 10.1188/16.CJON.S1.2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francipane M.G., Douradinha B., Chinnici C.M., Russelli G., Conaldi P.G., Iannolo G. Zika virus: a new therapeutic Candidate for glioblastoma treatment. Int. J. Mol. Sci. 2021;22(20) doi: 10.3390/ijms222010996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yadav B., Pal S., Rubstov Y., Goel A., Garg M., Pavlyukov M., Pandey A.K. LncRNAs associated with glioblastoma: from transcriptional noise to novel regulators with a promising role in therapeutics. Mol. Ther. Nucleic Acids. 2021;24:728–742. doi: 10.1016/j.omtn.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashrafizadeh M., Zarabi A., Hushmandi K., Moghadam E.R., Hashemi F., Daneshi S., Hashemi F., Tavakol S., Mohammadinejad R., Najafi M., Dudha N., Garg M. C-Myc signaling pathway in treatment and prevention of brain tumors. Curr. Cancer Drug Targets. 2021;21:2–20. doi: 10.2174/1568009620666201016121005. [DOI] [PubMed] [Google Scholar]
- 7.Marx S., Wilken F., Wagner I., Marx M., Troschke-Meurer S., Zumpe M., Bien-Moeller S., Weidemeier M., Baldauf J., Fleck S.K., Rauch B.H., Schroeder H.W.S., Lode H., Siebert N. GD2 targeting by dinutuximab beta is a promising immunotherapeutic approach against malignant glioma. J. Neurooncol. 2020;147:577–585. doi: 10.1007/s11060-020-03470-3. [DOI] [PubMed] [Google Scholar]
- 8.Straathof K., Flutter B., Wallace R., Jain N., Loka T., Depani S., Wright G., Thomas S., Cheung G.W., Gileadi T., Stafford S., Kokalaki E., Barton J., Marriott C., Rampling D., Ogunbiyi O., Akarca A.U., Marafioti T., Inglott S., Gilmour K., Al-Hajj M., Day W., McHugh K., Biassoni L., Sizer N., Barton C., Edwards D., Dragoni I., Silvester J., Dyer K., Traub S., Elson L., Brook S., Westwood N., Robson L., Bedi A., Howe K., Barry A., Duncan C., Barone G., Pule M., Anderson J. Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abd6169. [DOI] [PubMed] [Google Scholar]
- 9.Prapa M., Chiavelli C., Golinelli G., Grisendi G., Bestagno M., Di Tinco R., Dall'Ora M., Neri G., Candini O., Spano C. GD2 CAR T cells against human glioblastoma. NPJ Precis. Oncol. 2021;5:1–14. doi: 10.1038/s41698-021-00233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marofi F., Motavalli R., Safonov V.A., Thangavelu L., Yumashev A.V., Alexander M., Shomali N., Chartrand M.S., Pathak Y., Jarahian M. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res. Ther. 2021;12:1–16. doi: 10.1186/s13287-020-02128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon D.H., Osborn M.J., Tolar J., Kim C.J. Incorporation of immune checkpoint blockade into chimeric antigen receptor T cells (CAR-Ts): combination or built-in CAR-T. Int. J. Mol. Sci. 2018;19:340. doi: 10.3390/ijms19020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An Z., Hu Y., Bai Y., Zhang C., Xu C., Kang X., Yang S., Li W., Zhong X. Antitumor activity of the third generation EphA2 CAR-T cells against glioblastoma is associated with interferon gamma induced PD-L1. Oncoimmunology. 2021;10 doi: 10.1080/2162402X.2021.1960728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey N., Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol. Blood Marrow Transpl. 2019;25:e123–e127. doi: 10.1016/j.bbmt.2018.12.756. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed M., Goldgur Y., Hu J., Guo H.F., Cheung N.K. In silico driven redesign of a clinically relevant antibody for the treatment of GD2 positive tumors. PLoS One. 2013;8:e63359. doi: 10.1371/journal.pone.0063359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y., Liu Z., Wang X., Wu H., Zhang J., Yang J., Zhang F., Liu L., Long J., Lu P. Treatment with humanized selective CD19CAR-T cells shows efficacy in highly treated B-ALL patients who have relapsed after receiving murine-based CD19CAR-T therapieshs CAR-T treatment on B-ALL patients who have received mCAR-T. Clin. Cancer Res. 2019;25:5595–5607. doi: 10.1158/1078-0432.CCR-19-0916. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Zhang C., Qiao M., Cheng C., Tang N., Lu S., Sun W. Depletion of BATF in CAR-T cells enhances antitumor activity by inducing resistance against exhaustion and formation of central memory cells. Cancer Cell. 2022;40:1407–1422. doi: 10.1016/j.ccell.2022.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z., Hambardzumyan D. Immune microenvironment in glioblastoma subtypes. Front. Immunol. 2018;9:1004. doi: 10.3389/fimmu.2018.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura K., Tanaka Y., Shitara K., Hanai N. Construction of humanized anti-ganglioside monoclonal antibodies with potent immune effector functions. Cancer Immunol. Immunother. 2001;50:275–284. doi: 10.1007/PL00006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasikumar P.G., Ramachandra M. Small-molecule immune checkpoint inhibitors targeting PD-1/PD-L1 and other emerging checkpoint pathways. BioDrugs. 2018;32:481–497. doi: 10.1007/s40259-018-0303-4. [DOI] [PubMed] [Google Scholar]
- 20.Majzner R.G, Ramakrishna S, Yeom K.W., Patel S., Chinnasamy H., Schultz L.M., Richards R.M. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603:934–941. doi: 10.1038/s41586-022-04489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer S.K., Williams K., Wang L., Capio E., Briman M. Development of an IL-6 point-of-care assay: utility for real-time monitoring and management of cytokine release syndrome and sepsis. Bioanalysis. 2019;11:1777–1785. doi: 10.4155/bio-2019-0192. [DOI] [PubMed] [Google Scholar]
- 22.Tedesco V.E., Mohan C. Biomarkers for predicting cytokine release syndrome following CD19-targeted CAR T cell therapy. J. Immunol. 2021;206:1561–1568. doi: 10.4049/jimmunol.2001249. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki M., Cheung N.-K.V. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin. Ther. Targets. 2015;19:349–362. doi: 10.1517/14728222.2014.986459. [DOI] [PubMed] [Google Scholar]
- 24.Nazha B., Inal C., Owonikoko T.K. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front. Oncol. 2020;10:1000. doi: 10.3389/fonc.2020.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salinas R.D., Durgin J.S., O'Rourke D.M. Potential of glioblastoma-targeted chimeric antigen receptor (CAR) T-cell therapy. CNS Drugs. 2020;34:127–145. doi: 10.1007/s40263-019-00687-3. [DOI] [PubMed] [Google Scholar]
- 26.Prinzing B.L., Gottschalk S.M., Krenciute G. CAR T-cell therapy for glioblastoma: ready for the next round of clinical testing? Expert Rev. Anticancer Ther. 2018;18:451–461. doi: 10.1080/14737140.2018.1451749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medikonda R., Dunn G., Rahman M., Fecci P., Lim M. A review of glioblastoma immunotherapy. J. Neurooncol. 2021;151:41–53. doi: 10.1007/s11060-020-03448-1. [DOI] [PubMed] [Google Scholar]
- 28.Grosser R., Cherkassky L., Chintala N., Adusumilli P.S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36:471–482. doi: 10.1016/j.ccell.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J., Wang Y., Shi J., Liu J., Li Q., Chen L. Combination therapy: a feasibility strategy for CAR‑T cell therapy in the treatment of solid tumors. Oncol. Lett. 2018;16:2063–2070. doi: 10.3892/ol.2018.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heczey A., Louis C.U., Savoldo B., Dakhova O., Durett A., Grilley B., Liu H., Wu M.F., Mei Z., Gee A. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 2017;25:2214–2224. doi: 10.1016/j.ymthe.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akhavan D., Alizadeh D., Wang D., Weist M.R., Shepphird J.K., Brown C.E. CAR T cells for brain tumors: lessons learned and road ahead. Immunol. Rev. 2019;290:60–84. doi: 10.1111/imr.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nellan A., Rota C., Majzner R., Lester-McCully C.M., Griesinger A.M., Mulcahy Levy J.M., Foreman N.K., Warren K.E., Lee D.W. Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J. Immunother. Cancer. 2018;6:1–14. doi: 10.1186/s40425-018-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adusumilli P.S., Cherkassky L., Villena-Vargas J., Colovos C., Servais E., Plotkin J., Jones D.R., Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).