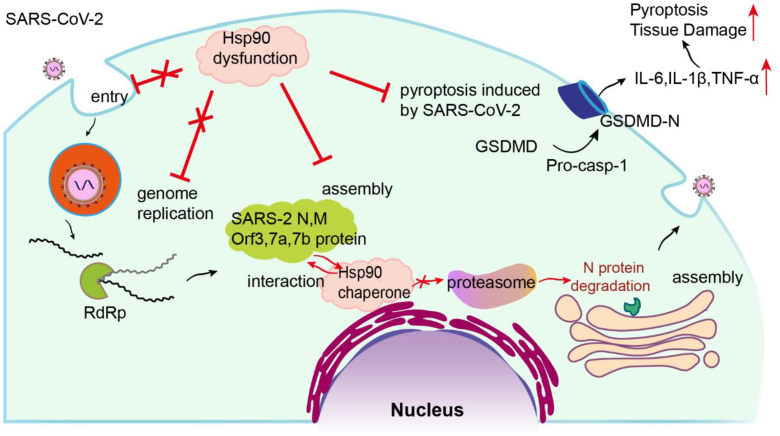
Figure 8.
Role of the Hsp90 network at various steps in the SARS-CoV-2 life cycle. Hsp90 was neither shown to chaperone the SARS-CoV-2 S protein, which is required for viral entry into target cells, nor did it chaperone the nonstructural proteins needed for viral replication. Hsp90 was found to chaperone N, M, Orf3, Orf7a, and Orf7b, and regulate virion assembly by preventing proteasomal degradation of N protein. Since SARS-CoV-2 infection promotes cleavage activation of caspase-1 and GSDMD, targeting Hsp90 can inhibit this process, reducing the subsequent hyperinflammation and pyroptosis that contribute to severe COVID-19 disease. COVID-19, coronavirus disease 2019; Hsp90, heat shock protein 90; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.