Abstract
Objectives
Subjective cognitive decline (SCD) is a known risk factor for Alzheimer’s disease. However, little research has examined whether healthy older adults with SCD (SCD+) exhibit lower cognition and increased rates of cognitive decline compared to those without SCD (SCD−). The goal of this study was to examine if cognitive change over a 15-year period differs between SCD+ and SCD−.
Method
3,019 cognitively normal older adults (831 SCD+) from 3 Rush Alzheimer’s Disease Center cohort studies were followed annually for up to a maximum of 15 years. Due to attrition, the average follow-up time was 5.7 years. Cognition was measured using z-scores of global cognition, episodic memory, semantic memory, perceptual speed, visuospatial ability, and working memory. Linear mixed-effects models investigated whether SCD was associated with cognitive change.
Results
Both baseline cognition and cognitive change over time differed between SCD+ and SCD−. People with SCD+ exhibited lower baseline scores and a steeper decline in global cognition, episodic memory, semantic memory, and perceptual speed. People with SCD+ did not differ from SCD− in baseline visuospatial ability or working memory but exhibited increased change over time in those two domains compared to SCD−.
Discussion
The observed results reveal that older adults with SCD+ have lower baseline cognition and steeper declines in cognition over time compared to SCD−. Older adults with SCD may be aware of subtle cognitive declines that occur over time in global cognition, episodic memory, semantic memory, perceptual speed, visuospatial ability, and working memory compared to those without SCD.
Keywords: Cognitive change, Cognitively healthy older adults, Subjective cognitive complaints
Alzheimer’s disease (AD) is a neurogenerative disease characterized by progressive declines in cognitive functioning that are severe enough to interfere with activities of daily living (Alzheimer’s Association, 2021). The onset of symptoms that result in a clinical diagnosis of AD occurs years, even decades, after the accumulation of amyloid plaques (amyloid β deposits) and neurofibrillary tangles (tau), and after neurodegeneration has begun (Femminella et al., 2018; Scheltens et al., 2016). This long phase between neuropathological development and clinical symptoms has become an area that has gained much attention as researchers attempt to identify biomarkers and other mechanisms of early detection for AD. Interestingly, many older adults who have no evidence of cognitive deficits on standardized tests report self-perceived concerns regarding declines in their cognitive abilities (Farias et al., 2018). As such, subjective cognitive decline (SCD) has emerged as an early risk factor for the development of future cognitive decline and AD (Jessen et al., 2014, 2020; Mitchell et al., 2014; Rabin et al., 2017).
The current research investigating the association between SCD and objective cognitive performance is not only limited but also contradictory. When examining studies that explore the relationship between SCD and cognition in terms of either baseline cross-sectional performance or longitudinal follow-ups, several have reported no differences between healthy older adults with SCD (+), and those without SCD (−) on a range of cognitive functions including memory, language, executive functioning, and global cognition (Carmasin et al., 2021; Lehrner et al., 2017; Yu et al., 2020). Conversely, several studies have observed that SCD+ is associated with lower cognitive performance in these same cognitive domains (i.e., memory, language, executive functioning, and global cognition) (Kim et al., 2020; Mulligan et al., 2016; Wolfsgruber et al., 2020). One study examined responses to a memory failure questionnaire using a “low,” “medium,” or “high” SCD approach depending on a number of questions endorsed (Carrasco et al., 2017). These authors found that those with higher SCD scores exhibited lower baseline global cognition and memory. A similar method was used in a longitudinal study reporting that greater severity of SCD predicted poorer cognition at baseline and at follow-up using bivariate analysis; however, SCD did not predict either baseline or cognitive decline when using linear mixed modeling (Sohrabi et al., 2019). Other longitudinal studies have been more consistent in observing that SCD is associated with greater cognitive decline. For example, greater declines in prospective memory (Kamberis et al., 2021), semantic fluency (Maruta & Martins, 2019), working memory (Vogel et al., 2017), verbal memory (Hohman et al., 2011), and episodic memory (Koppara et al., 2015) have all been observed in people with SCD+ compared to SCD− (see Rabin et al., 2017 for a review of more longitudinal studies). Therefore, while cross-sectional and short-term follow-up studies (i.e., 1–2 years) are inconclusive, several longitudinal studies (i.e., approximate follow-up time of 7 years) have noted that SCD is associated with subsequent declines in various cognitive domains over time.
Changes in the preclinical AD stage are thought to occur on average around 10 years before dementia diagnosis (Jessen et al., 2020); and around 60% of people with SCD convert to MCI and AD over a 15-year period (Reisberg & Gauthier, 2008). Therefore, although longitudinal studies of less than 10 years may capture differences in cognitive abilities between SCD+ and SCD−, the changes in cognitive function may reflect years of pathological progression of disease in those with SCD. Given the current push for earlier detection to aid in disease management and care planning, it is important to capture these individuals early in the disease process when SCD is present, but objective cognitive decline is not. Studies examining SCD are limited in terms of more extensive follow-up times (e.g., 10–15 years); however, tracking individuals reporting SCD early in the disease process is essential to understand progression of cognitive decline. In addition to this limitation in the current research, as noted above, few longitudinal studies have examined the influence of SCD on multiple cognitive domains (see Rabin et al. [2017] for review). That is, most studies focus on examining differences in cognition using one or two domains (e.g., memory and language) rather than a more extensive cognitive battery.
Examining only one, or a couple cognitive domains, rather than a range of domains, reduces the comparability across studies because most studies use different methods to quantify SCD in their participant population. For example, Maruta & Martins (2019) quantified SCD based on only language complaints. These language complaints were associated with worse performance on semantic fluency but not on memory, executive functioning, attention, or processing speed. Another study categorized SCD using a single question “Do you have difficulty with your memory?” Those who endorsed the question were then followed up with a questionnaire to determine SCD severity (Sohrabi et al., 2019). These authors observed that SCD severity was associated with current and future cognitive functioning. With studies examining different cognitive domains and using different SCD questionnaires, it is difficult to generalize the results and compare findings across the different studies. These various results highlight the novelty and importance of the current study; a longitudinal method with a 15-year follow-up, that uses one questionnaire to define SCD, and assesses multiple cognitive domains in the same population.
The current study was designed to leverage the harmonized Rush cohort studies which have extensive follow-up times to examine cognitive differences between healthy older adults with SCD and those without SCD both cross-sectionally, as well as longitudinally, over a period of 15 years. Two earlier studies from these cohorts examined the influence of depressive symptoms on memory complaints (Hill et al., 2020) and the influence of SCD on cognitive decline over 8 years (Arvanitakis et al., 2018). While Hill et al. (2020) observed an association between depressive symptoms and memory complaints, they did not examine this association with respect to cognition or cognitive decline. Arvanitakis et al. (2018) examined the association of memory complaints with cognition in a sample of healthy older adults and people with baseline MCI and dementia. However, to examine the preclinical dementia period and investigate early signs of detection, research needs to examine memory complaints in healthy older adults prior to the manifestation of clinical symptoms. Given that pathological brain changes occur up to 15–20 years before the presentations of clinical symptoms (Villemagne et al., 2013), examining healthy older adults over at least 10 years will improve our understanding of the association between SCD and cognitive decline.
In this study, examining cognitively healthy older adults over a 15-year follow-up period, we investigated the influence of SCD on global cognition, episodic memory, semantic memory, perceptual speed, working memory, and perceptual orientation/visuospatial ability. Given the previously reported relationship between SCD and depression (Hill et al., 2020; Rabin et al., 2017), analyses were run with and without the depressive symptom scores as a covariate to ensure that results were not influenced by depressive symptoms. The goal of this project was to determine: (a) if SCD is associated with current and future cognitive decline, (b) if cognitive decline is domain specific, and (c) whether the results are influenced by depressive symptom scores.
Method
Participants
Data used in the preparation of this article were obtained from the RADC Research Resource Sharing Hub (www.radc.rush.edu). Participants provided informed written consent to participate in one of three cohort studies on aging and dementia: (a) Minority Aging Research Study (MARS, Barnes et al., 2012), (b) Rush Alzheimer’s Disease Center African American Clinical Core (RADC AA Core, Schneider et al., 2009), or (c) the Rush Memory and Aging Project (MAP, Bennett et al., 2018).
Participant inclusion criteria for this specific study were as follows: (a) cognitively healthy status at their baseline visit, (b) no report of stroke, (c) had at least two cognitive assessments, (d) completed the questionnaire assessing memory complaints, and (e) were at least 55 years of age at baseline. A clinical diagnosis of cognitive status was completed using a three-stage process including computer scoring of cognitive tests, clinical judgment by a neuropsychologist, and diagnostic classification by a clinician based on criteria of the joint working group of the National Institute on Aging and the Alzheimer’s Association (NIA-AA) (McKhann et al., 2011). A total of 3,019 healthy older adult (NC = 2,188; SCD = 831) participants with a mean follow-up time of 5.7 years (and a total of 24,689 follow-ups available for analysis) were included in this study. Follow-ups were completed annually, for a maximum period of 28 years; however, for the purposes of this study, we used a maximum of 15 years because the sample size after 15 years in people with SCD was reduced to less than 100 participants.
Subjective cognitive decline was defined based on two questions examining memory complaints. Participants were asked, “About how often do you have trouble remembering things?” and “Compared to 10 years ago, would you say that your memory is much worse, a little worse, the same, a little better, or much better?”. Both questions were scored using a scale of 1–5 with 1 being never/much better and 5 being often/worse. Following past research and the Rush recommendations, if the participants had a composite score of 8–10 on these two questions they were classified as having memory complaints (Arvanitakis et al., 2018); reported as subjective cognitive decline (SCD+) in this paper.
Depressive symptoms were assessed with a 10-item modified version of the Center for Epidemiologic Studies Depression scale (CES-D, Kohout et al., 1993). Participants were asked Yes/No questions regarding whether or not they experienced each of the ten symptoms in the past week. Scores for each question ranged from 0 to 3 with 0 being rarely and 3 being most. The score was calculated based on the total number of items experienced. The total score ranges from 0 to 30, with higher scores indicating more depressive symptoms.
Cognitive Scores
A neurological battery comprised of 19 cognitive assessments was administered to all participants annually (Barnes et al., 2012). Five cognitive domains were assessed through the selected 19 tests: episodic memory, semantic memory, working memory, visuospatial ability, and perceptual speed. Episodic memory was assessed through scores from Word List Memory, Word List Recall, Word List Recognition, and immediate and delayed recall scores of both Story A on Logical Memory and the East Boston Story. Semantic memory was assessed through performance on Verbal Fluency, and on both a 15-item reading test, and version of the Boston Naming Test. The working memory domain was measured through performance on Digit ordering, as well as Digit Span Forwards and Backwards. Visuospatial ability was assessed through performance on a 16-item version of the Progressive Matrices and a 15-item version of Judgement and Line Orientation. Perceptual speed was measured through performance on Number Comparison, two indices from a modified version of the Stroop Neuropsychological Screening Test, and the Symbol Digit Modalities Test. Raw scores on each of the individual tests were converted to z-scores using the baseline mean (SD); and the z-scores of the tests from each domain were then averaged. An individual’s standard performance across all 19 of these tests was averaged to create a measure of global cognitive function (Lamar et al., 2020). More information for the specific tests used for each category can be obtained from https://www.radc.rush.edu/.
Statistical Analysis
Analyses were performed using R (R Core Team, 2021). Independent sample t-tests were completed on age, education, and baseline cognitive scores, and a chi-square analysis was completed to determine if the ratio of males to females differed between the groups. Multiple comparisons were corrected for using Bonferroni correction. Demographic information and baseline cognitive composite scores are presented in Table 1. Cognitive differences between SCD+ and SCD− were investigated using linear mixed-effects models to examine the association between each cognitive domain (global, episodic memory, semantic memory, perceptual speed, working memory, and visuospatial abilities). Linear mixed-effects model results were corrected for multiple comparisons using false discovery rate (FDR). p-values were reported as raw values with significance, then determined by FDR correction.
Table 1.
Demographic Characteristics of Cognitively Healthy Older Adults
| Demographic information | SCD− | SCD+ |
|---|---|---|
| (n = 2,188) | (n = 831) | |
| Age | 75.10 ± 7.18 | 76.4 ± 7.4* |
| Education | 15.9 ± 4.1 | 15.7 ± 3.9 |
| Male Sex | 528 (24%) | 196 (24%) |
| CES-D Score | 0.89 ± 1.44 | 1.50 ± 1.88* |
| Baseline global cognition | 0.27 ± 0.46 | 0.20 ± 0.44* |
| Baseline episodic memory | 0.34 ± 0.49 | 0.23 ± 0.48* |
| Baseline semantic memory | 0.28 ± 0.67 | 0.18 ± 0.65* |
| Baseline perceptual speed | 0.28 ± 0.76 | 0.16 ± 0.72* |
| Baseline visuospatial abilities | 0.22 ± 0.70 | 0.23 ± 0.67 |
| Baseline working memory | 0.16 ± 0.75 | 0.15 ± 0.78 |
| Cohort information | ||
| CORE | 186 (8.5%) | 68 (8%) |
| LATC | 99 (4.5%) | 49 (6%) |
| MAP | 857 (39%) | 354 (43%) |
| MARS | 378 (17%) | 141 (17%) |
| ROS | 668 (31%) | 219 (26%) |
| White race | 1,485 (68%) | 550 (66%) |
| Black race | 621 (28%) | 234 (28%) |
| Other race | 82 (4%) | 47 (6%) |
Notes: Scores are presented as mean ± standard deviation or number of sample and percentage of population. CES-D = Center for Epidemiologic Studies Depression scale; CORE = Clinical CORE Study; LATC = Latino CORE Study; MAP = Memory and Aging Project; MARS = Minatory Aging Research Study; ROS = Religious Orders Study; SCD+ = cognitively healthy older adults with subjective cognitive decline; SCD− = cognitively healthy older adults without subjective cognitive decline.
*Represents values that were significantly different between the groups.
The categorical variable of interest was diagnosis (i.e., SCD+ vs. SCD−) based on endorsement of memory complaints, contrasting the SCD+ cohort against SCD−. The models also included time from baseline, sex, years of education, and age at baseline as covariates. Participant ID was included as a categorical random effect to account for repeated measures of the same participant. Time from baseline was also included as a random effect to help account for random missing data points throughout the follow-up period.
| (1) |
A second model was included using the same variables, but including depressive symptoms as a covariate.
| (2) |
Results
Demographics
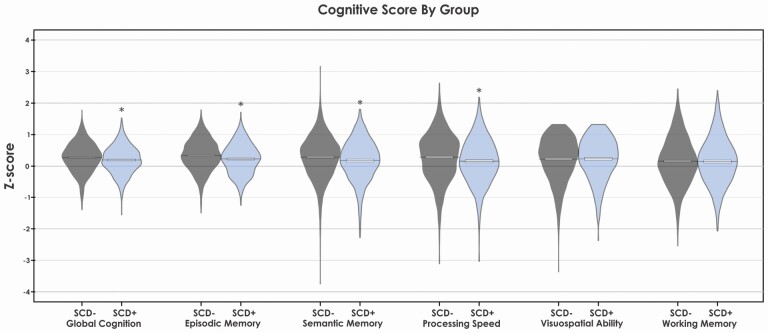
Table 1 summarizes demographic and baseline cognitive scores for all participants included in this study. Figure 1 shows plots for baseline cognitive scores for both SCD+ and SCD− participants. SCD+ participants were significantly older (p < .001), had more depressive symptoms on the CES-D (p < .001), but did not differ in years of education or number of males than SCD−. SCD+ also exhibited lower baseline scores on global cognition, episodic memory, semantic memory, and perceptual speed. This study consisted of three main cohorts, which contains data from five different data sets. These data sets were collected to be racially diverse. For comparison purposes, the number and percentage of participants from each data set were included in the demographic information to show a similar distribution of participants across the groups from all data sets. Furthermore, we included the number and percentage of White, Black, and Other to show the similar distribution of race across groups and data sets.
Figure 1.
Baseline cognitive scores for all domains by group. Notes: SCD+ = healthy older adults with subjective cognitive decline. SCD− = healthy older adults without subjective cognitive decline. * Represents cognitive domains that were significantly different at baseline between the groups using independent samples t-test and corrected using Bonferroni correction.
Demographic information is provided for the baseline sample, the full longitudinal sample, and dropouts in Supplementary Table 1. The full longitudinal sample at maximum follow-up had more years of education (p < .001), were younger (p < .001), had fewer depressive symptoms (p < .001), and higher global cognition (p < .001), episodic memory (p < .001), semantic memory (p < .001), perceptual speed (p < .001), visuospatial abilities (p < .001), and baseline working memory (p < .001) compared to the baseline sample.
Cognitive Outcomes
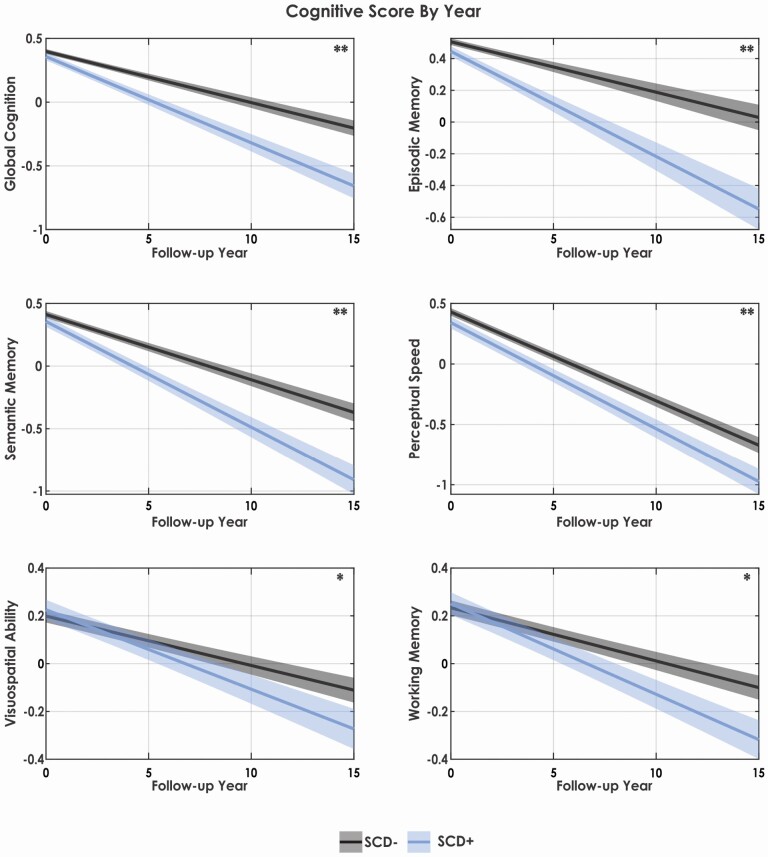
Table 2 summarizes the results of the linear mixed-effects models for all cognitive domains for both SCD+ and SCD−. Figure 2 presents the cognitive change over time by group as predicted by the linear mixed effects models. For all cognitive domains (i.e., global cognition, episodic memory, semantic memory, perceptual speed, visuospatial abilities, and working memory), increased age and follow-up year were associated with lower cognitive performance (p < .001), whereas increased education was associated with improved cognitive performance (p < .001). Males exhibited lower global cognition, episodic memory, semantic memory, and perceptual speed (p < .005), but exhibited higher visuospatial abilities (p < .001) than females.
Table 2.
Linear Mixed-Effects Models Examining Influence of SCD+ Status on Cognition Over Time
| Global cognition | Episodic memory | Semantic memory | Perceptual speed | Visuospatial abilities | Working memory | |
|---|---|---|---|---|---|---|
| Age | β = −0.01 | β = −0.02 | β = −0.02 | β = −0.03 | β = −0.007 | β = −0.004 |
| SE = 0.001 | SE = 0.001 | SE = 0.001 | SE = 0.001 | SE = 0.001 | SE = 0.002 | |
| t = −13.05, p < .001* | t = −13.37, p < .001* | t = −12.55, p < .001* | t = −18.92, p < .001* | t = −5.22, p < .001* | t = −2.85, p < .001* | |
| Time From Baseline | β = −0.04 | β = −0.03 | β = −0.05 | β = −0.07 | β = −0.02 | β = −0.02 |
| SE = 0.002 | SE = 0.002 | SE = 0.002 | SE = 0.002 | SE = 0.001 | SE = 0.001 | |
| t = −19.93, p < .001* | t = −11.72, p < .001* | t = −21.43, p < .001* | t = −34.60, p < .001* | t = −11.81, p < .001* | t = −13.04, p < .001* | |
| SCD Diagnosis | β = −0.04 | β = −0.06 | β = −0.06 | β = −0.09 | β = 0.03 | β = 0.02 |
| SE = 0.02 | SE = 0.02 | SE = 0.02 | SE = 0.03 | SE = 0.02 | SE = 0.03 | |
| t = −2.36, p = .018* | t = −3.27, p = .001* | t = −2.28, p = .022* | t = −3.19, p = .001* | t = −1.11, p = .27 | t = 0.64, p = .52 | |
| SCD: Time From Baseline | β = −0.03 | β = −0.03 | β = −0.03 | β = −0.01 | β = −0.01 | β = −0.02 |
| SE = 0.003 | SE = 0.005 | SE = 0.004 | SE = 0.004 | SE = 0.003 | SE = 0.003 | |
| t = −7.19, p < .001* | t = −6.67, p < .001* | t = −6.96, p < .001* | t = −3.53, p < .001* | t = −3.74, p < .001* | t = −4.79, p < .001* | |
| Male Sex | β = −0.09 | β = −0.17 | β = −0.07 | β = −0.20 | β = 0.26 | β = −0.03 |
| SE = 0.02 | SE = 0.02 | SE = 0.03 | SE = 0.03 | SE = 0.02 | SE = 0.03 | |
| t = −4.96, p < .001* | t = −9.03, p < .001* | t = −2.96, p = .003* | t = −7.11, p < .001* | t = 10.90, p < .001* | t = −1.04, p = .30 | |
| Education | β = 0.06 | β = 0.05 | β = 0.05 | β = 0.07 | β = 0.06 | β = 0.06 |
| SE = 0.002 | SE = 0.002 | SE = 0.002 | SE = 0.003 | SE = 0.003 | SE = 0.003 | |
| t = 29.51, p < .001* | t = 22.15, p < .001* | t = 19.01, p <. 001* | t = 21.74, p < .001* | t = 22.29, p < .001* | t = 21.74, p < .001* |
Notes: SCD = subjective cognitive decline.
*Represents the values that were statistically significant. All results remained significant after correction for multiple comparisons using false discovery rate.
A main effect of SCD diagnosis was obtained for global cognition, episodic memory, semantic memory, and perceptual speed (but not visuospatial abilities or working memory). An interaction between SCD and follow-up year was obtained for all cognitive domains (i.e., global cognition, episodic memory, semantic memory, perceptual speed, visuospatial abilities, and working memory). For global cognition, both a main effect of SCD+ (p = .018) and an interaction between SCD and follow-up year was observed (p < .001). For episodic memory, the results showed a main effect of SCD (p = .001) and an interaction between SCD and follow-up year (p < .001). Semantic memory was also associated with both a main effect of SCD+ (p = .022) and an interaction between SCD and follow-up year (p < .001). Perceptual speed showed a main effect of SCD (p = .004) and interaction between SCD and follow-up year (p < .001). Both visuospatial abilities and working memory had no main effect of SCD, but a significant interaction between SCD and follow-up for both visuospatial abilities (p < .001) and working memory (p < .001) was observed. These results suggest that not only do those with SCD have lower performance on tests of global cognition, episodic, semantic memory, and perceptual speed, but that they also have increased rates of decline over time compared to those without SCD. Those with SCD do not show lower working memory and visuospatial abilities at baseline, but have increased rates of decline in these two domains over time compared to those without SCD.
Cognitive Outcomes Controlling for Depressive Symptoms and Other Covariates
Table 3 summarizes the linear mixed-effects model results for all cognitive domains for both SCD+ and SCD− when controlling for depressive symptoms. All results remained significant with and without depressive symptoms as a covariate. Depressive symptoms were negatively associated with all cognitive domains: global cognition (p < .001), episodic memory (p < .001), semantic memory (p < .001), perceptual speed (p < .001), visuospatial abilities (p < .001), and working memory (p < .001).
Table 3.
Linear Mixed-Effects Models Examining Influence of SCD+ Status on Cognition Over Time Controlling for Depression
| Global cognition | Episodic memory | Semantic memory | Perceptual speed | Visuospatial abilities | Working memory | |
|---|---|---|---|---|---|---|
| Age | β = −0.01 | β = −0.02 | β = −0.02 | β = −0.03 | β = −0.008 | β = −0.004 |
| SE = 0.001 | SE = 0.001 | SE = 0.001 | SE = 0.001 | SE = 0.001 | SE = 0.002 | |
| t = −24.31, p < .001* | t = −14.12, p < .001* | t = −13.53, p<.001* | t = −19.24, p < .001* | t = −5.71, p < .001* | t = −3.16, p = .001* | |
| Time from baseline | β = −0.04 | β = −0.03 | β = −0.05 | β = −0.07 | β = −0.02 | β = −0.02 |
| SE = 0.001 | SE = 0.002 | SE = 0.002 | SE = 0.002 | SE = 0.001 | SE = 0.001 | |
| t = −20.59, p < .001* | t = −12.09, p < .001* | t = −22.62, p < .001* | t = −31.28, p < .001* | t = −12.47, p < .001* | t = −13.16, p <.001* | |
| SCD diagnosis | β = −0.03 | β = −0.05 | β = −0.05 | β = −0.07 | β = 0.03 | β = 0.02 |
| SE = 0.02 | SE = 0.02 | SE = 0.02 | SE = 0.03 | SE = 0.02 | SE = 0.03 | |
| t = −1.95, p = .05 | t = −2.60, p =.009* | t = −2.27, p = .023* | t = −2.67, p = .007* | t = 1.30, p = .19 | t = 0.77, p =.44 | |
| SCD: time from baseline | β = −0.03 | β = −0.03 | β = −0.03 | β = −0.01 | β = −0.01 | β = −0.15 |
| SE = 0.003 | SE = 0.005 | SE = 0.005 | SE = 0.004 | SE = 0.003 | SE = 0.003 | |
| t = −7.11, p < .001* | t = −6.63, p < .001* | t = −6.69, p < .001* | t = −3.48, p < .001* | t = −3.49, p < .001* | t = −4.60, p < .001* | |
| Male Sex | β = −0.10 | β = −0.19 | β = −0.10 | β = −0.21 | β = 0.26 | β = −0.03 |
| SE=0.02 | SE = 0.019 | SE = 0.025 | SE = 0.03 | SE = 0.02 | SE = 0.03 | |
| t = −5.87, p < .001* | t = −9.70, p < .001* | t = −3.94, p < .001* | t = −7.50, p < .001* | t = 10.70, p < .001* | t = −1.28, p = .20 | |
| Education | β = 0.05 | β = 0.04 | β = 0.05 | β = 0.07 | β = 0.06 | β = 0.06 |
| SE = 0.002 | SE = 0.002 | SE = 0.002 | SE = 0.003 | SE = 0.003 | SE = 0.003 | |
| t = 28.90, p < .001* | t = 21.32, p < .001* | t = 18.39, p < .001* | t = 21.56, p < .001* | t = 21.75, p < .001* | t = 21.45, p < .001* | |
| Depressive symptoms | β = −0.02 | β = −0.02 | β = −0.02 | β = −0.03 | β = −0.02 | β = −0.01 |
| SE = 0.002 | SE = 0.002 | SE = 0.002 | SE = 0.002 | SE = 0.003 | SE = 0.003 | |
| t = −12.62, p < .001* | t = −9.94, p < .001* | t = −7.90, p < .001* | t = −12.05, p < .001* | t = −7.20, p < .001* | t = −5.59, p < .001* |
Notes: SCD = subjective cognitive decline.
*Represents the values that were statistically significant. Bold values represent results that were no longer significant after correction for multiple comparison and that differed from the analysis not including depressive symptoms.
All results remained the same compared to the initial analysis, except for the main effect of SCD no longer being significant for global cognition. It should also be noted that all models were repeated, including APOE-ε4 positivity and personality traits as additional covariates. All results that were significant in the original models remained significant with the additional covariates. That is, the models with the additional covariates produced the same results in terms of effect size and significance, reflecting an insignificant role of both APOE-ε4 positivity and personality traits on the distinction between SCD+ and SCD− in the current study. We thus opted to include results from the models that did not include these risk factors.
Discussion
Previous research yields conflicting evidence as to whether healthy older adults with SCD exhibit lower cognition compared to healthy older adults without SCD. To address these inconsistencies in the field, the current study was designed to investigate whether SCD predicted baseline cognition and 15-year cognitive change. Findings from the current study reveal that SCD+ demonstrates lower baseline cognitive functioning in global cognition, episodic memory, semantic memory, and perceptual speed, and greater rates of cognitive change (decline) over time in all domains compared to SCD−. Although all participants are considered cognitively normal by objective neuropsychological testing, SCD+ exhibit significantly diminished cognitive functioning compared to their SCD− counterparts.
Although age is a primary risk factor for the development of neurodegenerative diseases like AD, there also exists a substantial body of literature highlighting the association between normal neurocognitive aging and cognitive decline (van Hooren et al., 2007). Consistent with this existing work, in our sample of cognitively normal healthy older adults, we observed that older age was associated with lower baseline cognition, and greater cognitive decline over time regardless of SCD status. Further, education was positively associated with cognitive performance suggesting that higher education is associated with enhanced cognition, and may be neuroprotective against cognitive decline; both of which have been extensively discussed in previous studies (Tucker & Stern, 2011). When controlling for both the risk factor of age and the protective factor of education in the present study to elucidate if cognitive differences existed as a function of SCD status, findings revealed that SCD+ demonstrated lower cognitive performance across a variety of cognitive domains at baseline. These findings suggest that SCD status may be associated with objective cognitive decline in individuals who are still considered cognitively normal from a clinical diagnostic standpoint. In addition, our data supports the hypothesis that SCD status is indicative of future cognitive decline. Across all cognitive domains observed in the present study, SCD+ was associated with significantly greater cognitive decline over time.
Current research examining cognition in SCD has been limited. Most studies focus on the influence of SCD on current or baseline cognition (e.g., Carmasin et al., 2021; Kim et al., 2020; Lehrner et al., 2017; Mulligan et al., 2016; Wolfsgruber et al., 2020; Yu et al., 2020). These studies have yielded mixed results as to whether SCD is associated with lower cognition. To expand on the extant work in order to address these mixed findings, we examined the influence of SCD on several cognition domains and observed that SCD+ status was associated with lower baseline performance in some (but not all) cognitive domains. This finding may contribute to why the aforementioned baseline/cross-sectional studies observed mixed results. SCD+ status may be associated with lower performance on specific cognitive domains, but not baseline cognition in general. Therefore, depending on the cognitive domains measured, studies may or may not observe group differences.
While observing more SCD+ group differences in cognition compared to baseline studies, previous longitudinal research is also inconsistent (e.g., Koppara et al., 2015; Vogel et al., 2017). In the current study, we found that SCD+ was associated with steeper declines in all cognitive domains measured compared to SCD−. These group differences were the largest in global cognition, episodic memory, and semantic memory. This finding is consistent with SCD+ being classified as an earlier indicator of future cognitive impairment (Rabin et al., 2017). It is well known that AD pathophysiology involves the build-up of amyloid-beta (Aβ) plaques and neurofibrillary tau tangles, resulting in neuronal injury and subsequent memory (and other cognitive domain) impairments as the disease progresses (Knopman et al., 2021; Scheltens et al., 2016). This AD-related pathophysiology begins decades prior to the development of clinical symptoms, during a preclinical AD stage. Therefore, explorations into the earliest noticeable signs of change are warranted, even if they are only subjective changes. The current findings presented in this paper support the hypothesis that SCD is an early indicator of future cognitive impairment and should consistently be assessed to track disease progression. Tracking SCD status may serve as an effective cost-efficient and noninvasive early indicator of cognitive decline, and future research should further examine progression to AD in people with SCD. We observed that SCD is associated with future cognitive decline in all domains assessed. It is thus likely that these complaints may be associated with early chemical changes in the brain resulting from abnormal Aβ protein levels evident in the initial stages of AD, prior to any observable objective cognitive change. Some research has observed that SCD is associated with increased atrophy (Morrison et al., 2022a), increased white matter hyperintensity burden (Morrison et al., 2022b), and increased levels of amyloid and tau in those who decline over time (Colijn & Grossberg, 2015). With a maximum follow-up of 8 years, those with SCD exhibited greater pathological AD and neurodegenerative pathologies (Arvanitakis et al., 2018). Future research should examine the association between these complaints and structural brain changes with 10–15-year follow-ups to examine preclinical AD changes in the earliest stage. Identifying the earliest pathological changes will improve selection of people for clinical trials, as well as treatment and prevention options.
Potential confounds within current research involve examining the association between SCD and depression. In the current study, we investigated depression as a covariate because several previous studies have revealed a positive association between depression and SCD (Brigola et al., 2015; Brown, Hill, & Haider, 2022; Markova, et al., 2017). Some results have suggested that depression explains differences in objective performance between SCD+ and SCD−, whereas others suggest that the relationship between SCD and cognition remains even when controlling for depression (see Burmester et al. [2016] for review). It is important to consider depression when investigating SCD because people with depression tend to have negative information processing biases where they selectively attend to negative information and have a heightened awareness for negative information (Beck, 2008), which in the case of cognitive aging, may manifest as an over-reporting of cognitive complaints (see Rabin et al. [2017] for review). Although previous research has observed that greater memory complaints are associated with more depressive symptoms (Hill et al., 2020), we found that even when controlling for depressive symptoms, the association between memory complaints and cognition remains.
Our results corroborate existing findings (Liew, 2019) and provide support for the notion that SCD and depression may function independently regarding their influence on the development of future cognitive decline. When controlling for depressive symptoms in our models, we observed that the main effect of SCD status on global cognition, episodic memory, and perceptual speed was no longer significant. In the current study, we observed that those with SCD had higher CES-D scores at baseline than those without SCD. Nevertheless, the rate of change over time remained steeper for those who were SCD+ for all cognitive domains. Our results thus align with both those suggesting depressive symptoms mitigate objective performance differences due to SCD, and those that report no group differences when controlling for depressive symptoms. These findings suggest that while depressive symptoms may influence baseline cognitive scores, change over time in those with SCD is not as strongly influenced by depressive symptoms. Several studies have also noted that depression may be associated with an increased risk for later cognitive decline and incipient dementia and AD (see Rabin et al. [2017] for review). Therefore, there may be an interaction effect between SCD and depression/depressive symptoms that further increases one’s risk for AD. Future research should further examine the individual and joint effects of SCD and depression on progression to AD.
Additionally, previous research suggests that although APOE ɛ4 positivity may be related to SCD and AD, there is limited evidence suggesting APOE ɛ4 positivity predisposes individuals to develop SCD (Ali et al., 2018). Similarly, we observed that APOE ɛ4 positivity did not change the significance of SCD main effects or interactions in the current study. This was also evident with personality; however, consistent with past research, we did find that personality traits had a significant main effect on SCD status (Studer et al., 2014). As such, future research may want to examine the combined influence of lifestyle (e.g., personality, APOE ɛ4 positivity, depression) and cognitive reserve factors (e.g., low vs. high education) on SCD status. This research would help improve our understanding of other influences on why SCD is associated with increased cognitive decline.
There are a few strengths and limitations of the current paper that should be noted. The current paper is novel in the design to examine future cognitive decline with a follow-up period of 15 years in a sample of only healthy older adults. In addition, our study had much larger samples than many of the past studies, which increases the confidence of this paper’s ability to detect subtle cognitive differences that occur in the SCD stage (i.e., preclinical AD), which may not be observable with fewer participants. A limitation of the current study is the education levels of the participants are high (M = 15.9 years, SD = 4.0 years) and thus may not be generalizable to other populations. In addition, although our sample was relatively diverse, we did not stratify our findings by race. It has been recently suggested that SCD may predict incident cognitive decline in Whites, but not in Blacks or Hispanics (Ferraro et al., 2022). As such, future research may wish to further investigate the relationship between SCD and incident cognitive decline in order to elucidate the role of SCD as a marker of early detection across individuals from diverse racial and ethnic backgrounds.
Previous research has observed that the use of different SCD questionnaires may lead to different clinical outcomes (Morrison et al., 2022a) and unique patterns of brain atrophy (Diaz-Galvan et al., 2021; Morrison et al., 2022a). It is thus possible that we did not observe changes in visuospatial abilities between SCD+ and SCD− because the questionnaire used here to operationalize SCD were not sensitive to changes in visuospatial abilities. Future research should examine the sensitivity of specific questionnaires to detect declines in all cognitive domains and develop a standardized method to classify SCD status in clinical settings.
Conclusion
This study examined the association between SCD and baseline cognition and future cognitive decline. The findings indicate that SCD status is associated with lower baseline global cognition, episodic memory, semantic memory, and perceptual speed, while being associated with future cognitive decline in all six domains. The association with steeper future cognitive decline in SCD+ compared to SCD− remains significant even after controlling for depressive symptoms. Taken together, these findings suggest that people with SCD may be aware of cognitive changes occurring before they are detectable on standardized cognitive tests. SCD may be predictive of decline up to 15 years out, and may be one of the earliest noticeable changes associated with early neuropathological change. This preclinical phase may be the best opportunity for early intervention before too much neuropathology damages the structural integrity of the brain resulting in objective cognitive performance declines that are measurable at an individual level. This study improves our current understanding of the preclinical phase of AD, which will increase the chances of earlier intervention and treatment options in those living with AD.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences online.
Figure 2.
Cognitive change over time for all domains by group and year. Notes: SCD+ = healthy older adults with subjective cognitive decline. SCD− = healthy older adults without subjective cognitive decline. ** Represents cognitive domains in which SCD+ exhibited both baseline differences and a steeper decline in cognition over time compared to SCD−. * Represents cognitive domains in which SCD+ exhibited only a steeper decline in cognition over time compared to SCD−.
Acknowledgments
We want to acknowledge all the MARS, AA Core, and MAP participants. We are also grateful for the hard work from the staff and investigators at the Rush Alzheimer’s Disease Center. To obtain data from MARS, AA Core, and MAP for research use, please visit the RADC Research Resource Sharing Hub (www.radc.rush.edu). This study was not preregistered.
Contributor Information
Cassandra Morrison, McConnell Brain Imaging Centre, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada; Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada.
Michael D Oliver, Department of Psychological Science and Neuroscience, Belmont University, Nashville, Tennessee, USA; Belmont Data Collaborative, Belmont University, Nashville, Tennessee, USA.
Funding
This work was supported by the National Institutes of Health, National Institute on Aging (R01 AG17917, R01 AG22018, P30 AG10161, and P30 AG72975). C. Morrison is supported by a postdoctoral fellowship from Canadian Institutes of Health Research, Funding Reference Number: MFE-176608. M. D. Oliver is supported by the Columbia Center for Interdisciplinary Research on Alzheimer’s Disease Disparities (GG013386-16).
Conflict of Interest
None declared.
References
- Ali, J. I., Smart, C. M., & Gawryluk, J. R. (2018). Subjective cognitive decline and APOE ɛ4: A systematic review. Journal of Alzheimer’s Disease, 65(1), 303–320. doi: 10.3233/jad-180248 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2021). What is Alzheimer’s disease. https://www.alz.org/alzheimers-dementia/what-is-alzheimers [Google Scholar]
- Arvanitakis, Z., Leurgans, S. E., Fleischman, D. A., Schneider, J. A., Rajan, K. B., Pruzin, J. J., Shah, R. C., Evans, D. A., Barnes, L. L., & Bennett, D. A. (2018). Memory complaints, dementia, and neuropathology in older blacks and whites. Annals of Neurology, 83(4), 718–729. doi: 10.1002/ana.25189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, L. L., Shah, R. C., Aggarwal, N. T., Bennett, D. A., & Schneider, J. A. (2012). The Minority Aging Research Study: Ongoing efforts to obtain brain donation in African Americans without dementia. Current Alzheimer Research, 9(6), 734–745. doi: 10.2174/156720512801322627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T. (2008). The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry, 165(8), 969–977. doi: 10.1176/appi.ajp.2008.08050721 [DOI] [PubMed] [Google Scholar]
- Bennett, D. A., Buchman, A. S., Boyle, P. A., Barnes, L. L., Wilson, R. S., & Schneider, J. A. (2018). Religious orders study and rush memory and aging project. Journal of Alzheimer’s Disease, 64(S1), S161–S189. doi: 10.3233/jad-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigola, A. G., Manzini, C. S. S., Oliveira, G. B. S., Ottaviani, A. C., Sako, M. P., & Vale, F. A. C. (2015). Subjective memory complaints associated with depression and cognitive impairment in the elderly: A systematic review. Dementia & Neuropsychologia, 9, 51–57. doi: 10.1590/S1980-57642015DN91000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M. J., Hill, N. L., & Haider, M. R. (2022). Age and gender disparities in depression and subjective cognitive decline-related outcomes. Aging & Mental Health, 26(1), 48–55. doi: 10.1080/13607863.2020.1861214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester, B., Leathem, J., & Merrick, P. (2016). Subjective cognitive complaints and objective cognitive function in aging: A systematic review and meta-analysis of recent cross-sectional findings. Neuropsychology Review, 26(4), 376–393. doi: 10.1007/s11065-016-9332-2 [DOI] [PubMed] [Google Scholar]
- Carrasco, P. M., Montenegro-Peña, M., López-Higes, R., Estrada, E., Crespo, D. P., Rubio, C. M., & Azorín, D. G. (2017). Subjective Memory Complaints in healthy older adults: Fewer complaints associated with depression and perceived health, more complaints also associated with lower memory performance. Archives of Gerontology and Geriatrics, 70, 28–37. doi: 10.1016/j.archger.2016.12.007 [DOI] [PubMed] [Google Scholar]
- Carmasin, J. S., Roth, R. M., Rabin, L. A., Englert, J. J., Flashman, L. A., & Saykin, A. J. (2021). Stability of subjective executive functioning in older adults with aMCI and subjective cognitive decline. Archives of Clinical Neuropsychology, 36(6), 1012–1018. doi: 10.1093/arclin/acaa129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colijn, M. A., & Grossberg, G. T. (2015). Amyloid and tau biomarkers in subjective cognitive impairment. Journal of Alzheimer’s Disease, 47(1), 1–8. doi: 10.3233/jad-150180 [DOI] [PubMed] [Google Scholar]
- Diaz-Galvan, P., Ferreira, D., Cedres, N., Falahati, F., Hernández-Cabrera, J. A., Ames, D., Barroso, J., & Westman, E. (2021). Comparing different approaches for operationalizing subjective cognitive decline: Impact on syndromic and biomarker profiles. Scientific Reports, 11(1), 1–15. doi: 10.1038/s41598-021-83428-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias, S. T., Giovannetti, T., Payne, B. R., Marsiske, M., Rebok, G. W., Schaie, K. W., Thomas, K. R., Willis, S. L., Dzierzewski, J. M., Unverzagt, F., & Gross, A. L. (2018). Self-perceived difficulties in everyday function precede cognitive decline among older adults in the ACTIVE study. Journal of the International Neuropsychological Society, 24(1), 104–112. doi: 10.1017/S1355617717000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femminella, G. D., Thayanandan, T., Calsolaro, V., Komici, K., Rengo, G., Corbi, G., & Ferrara, N. (2018). Imaging and molecular mechanisms of Alzheimer’s disease: A review. International Journal of Molecular Sciences, 19(12), 3702. doi: 10.3390/ijms19123702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro, K. F., Sauerteig-Rolston, M. R., Barnes, L. L., Friedman, E., Sands, L. P., & Thomas, P. A. (2022). Subjective memory decline predicts incident cognitive impairment among White—but not Black or Hispanic—older adults. The Gerontologist, gnac086. doi: 10.1093/geront/gnac086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, N. L., Mogle, J., Bhargava, S., Whitaker, E., Bhang, I., Capuano, A. W., Arvanitakis, Z., Bennett, D. A., & Barnes, L. L. (2020). Differences in the associations between memory complaints and depressive symptoms among Black and White older adults. The Journals of Gerontology: Series B, 75(4), 783–791. doi: 10.1093/geronb/gby091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman, T. J., Beason-Held, L. L., Lamar, M., & Resnick, S. M. (2011). Subjective cognitive complaints and longitudinal changes in memory and brain function. Neuropsychology, 25(1), 125–30. doi: 10.1037/a0020859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen, F., Amariglio, R. E., Buckley, R. F., van der Flier, W. M., Han, Y., Molinuevo, J. L., Rabin, L., Rentz, D. M., Rodriguez-Gomez, O., Saykin, A. J., Sikkes, S. A., Smart, C. M., Wolfsgruber, S., & Wagner, M. (2020). The characterisation of subjective cognitive decline. The Lancet Neurology, 19(3), 271–278. doi: 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen, F., Amariglio, R. E., Van Boxtel, M., Breteler, M., Ceccaldi, M., Chételat, G., Dubois, B., Dufouil, C., Ellis, K. A., van der Flier, W. M., Glodzik, L., van Harten, A. C., de Leon, M. J., McHugh, P., Mielke, M. M., Molinuevo, J. L., Mosconi, L., Osorio, R. S., & Perrotin, A., … & Subjective Cognitive Decline Initiative. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 10(6), 844–852. doi: 10.1016/j.jalz.2014.01.001.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamberis, N., Cavuoto, M. G., & Pike, K. E. (2021). The influence of subjective cognitive decline on prospective memory over 5 years. Neuropsychology, 35(1), 78–89. doi: 10.1037/neu0000709 [DOI] [PubMed] [Google Scholar]
- Kim, W. H., Kim, B. S., Chang, S. M., Lee, D. W., & Bae, J. N. (2020). Relationship between subjective memory complaint and executive function in a community sample of South Korean elderly. Psychogeriatrics, 20(6), 850–857. doi: 10.1111/psyg.12592 [DOI] [PubMed] [Google Scholar]
- Knopman, D. S., Amieva, H., Petersen, R. C., Chételat, G., Holtzman, D. M., Hyman, B. T., Nixon, R. A., & Jones, D. T. (2021). Alzheimer disease. Nature Reviews Disease Primers, 7(1), 1–21. doi: 10.1038/s41572-021-00269-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout, F. J., Berkman, L. F., Evans, D. A., & Cornoni-Huntley, J. (1993). Two shorter forms of the CES-D depression symptoms index. Journal of Aging and Health, 5(2), 179–193. doi: 10.1177/089826439300500202 [DOI] [PubMed] [Google Scholar]
- Koppara, A., Wagner, M., Lange, C., Ernst, A., Wiese, B., König, H. H., Brettschneider, C., Riedel-Heller, S., Luppa, M., Weyerer, S., Werle, J., Bickel, H., Mösch, E., Pentzek, M., Fuchs, A., Wolfsgruber, S., Beauducel, A., Scherer, M., & Jessen, F. (2015). Cognitive performance before and after the onset of subjective cognitive decline in old age. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 1(2), 194–205. doi: 10.1016/j.dadm.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar, M., Wilson, R. S., Yu, L., Stewart, C. C., Bennett, D. A., & Boyle, P. A. (2020). Associations of decision making abilities with blood pressure values in older adults. Journal of Hypertension, 38(1), 59–64. doi: 10.1097/HJH.0000000000002220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrner, J., Coutinho, G., Mattos, P., Moser, D., Pflüger, M., Gleiss, A., Auff, E., Dal-Bianco, P., Pusswald, G., & Stögmann, E. (2017). Semantic memory and depressive symptoms in patients with subjective cognitive decline, mild cognitive impairment, and Alzheimer’s disease. International Psychogeriatrics, 29(7), 1123–1135. doi: 10.1017/S1041610217000394 [DOI] [PubMed] [Google Scholar]
- Liew, T. M. (2019). Depression, subjective cognitive decline, and the risk of neurocognitive disorders. Alzheimer’s Research & Therapy, 11(1), 1–8. doi: 10.1186/s13195-019-0527-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markova, H., Andel, R., Stepankova, H., Kopecek, M., Nikolai, T., Hort, J., Thomas-Antérion, C., & Vyhnalek, M. (2017). Subjective cognitive complaints in cognitively healthy older adults and their relationship to cognitive performance and depressive symptoms. Journal of Alzheimer’s Disease, 59(3), 871–881. doi: 10.3233/jad-160970 [DOI] [PubMed] [Google Scholar]
- Maruta, C., & Martins, I. P. (2019). May subjective language complaints predict future language decline in community-dwelling subjects?. Frontiers in Psychology, 10, 1974. doi: 10.1093/geront/gnac086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack Jr, C. R., Kawas, C. H., Klunk, W. E., Koroshetz, W. J., Manly, J. J., Mayeux, R., Mohs, R. C., Morris, J. C., Rossor, M. N., Scheltens, P., Carrillo, M. C., Thies, B., Weintraub, S., & Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, A. J., Beaumont, H., Ferguson, D., Yadegarfar, M., & Stubbs, B. (2014). Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatrica Scandinavica, 130(6), 439–451. doi: 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- Morrison, C., Dadar, M., Shafiee, N., Villeneuve, S., & Collins, D. L.; Alzheimer’s Disease Neuroimaging Initiative. (2022a). Regional brain atrophy and cognitive decline depend on definition of subjective cognitive decline. NeuroImage: Clinical, 33, 102923. doi: 10.1016/j.nicl.2021.102923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, C., Dadar, M., Villeneuve, S., Ducharme, S., & Collins, D. L. (2022b). White matter hyperintensity load varies depending on subjective cognitive decline criteria. GeroScience . doi: 10.1007/s11357-022-00684-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan, B. P., Smart, C. M., & Ali, J. I. (2016). Relationship of subjective and objective performance indicators in subjective cognitive decline. Psychology & Neuroscience, 9(3), 362. doi: 10.1037/pne0000061 [DOI] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Rabin, L. A., Smart, C. M., & Amariglio, R. E. (2017). Subjective cognitive decline in preclinical Alzheimer’s disease. Annual Review of Clinical Psychology, 13, 369–396. doi: 10.1146/annurev-clinpsy-032816-045136 [DOI] [PubMed] [Google Scholar]
- Reisberg, B., & Gauthier, S. (2008). Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. International Psychogeriatrics, 20(1), 1–16. doi: 10.1017/S1041610207006412 [DOI] [PubMed] [Google Scholar]
- Scheltens, P., Blennow, K., Breteler, M. M., de Strooper, B., Frisoni, G. B., Salloway, S., & Van der Flier, W. M. (2016). Alzheimer’s disease. The Lancet, 388, 505–517. doi: 10.1016/S0140-6736(15)01124-1 [DOI] [PubMed] [Google Scholar]
- Schneider, J. A., Aggarwal, N. T., Barnes, L., Boyle, P., & Bennett, D. A. (2009). The neuropathology of older persons with and without dementia from community versus clinic cohorts. Journal of Alzheimer’s Disease, 18(3), 691–701. doi: 10.3233/jad-2009-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi, H. R., Weinborn, M., Laske, C., Bates, K. A., Christensen, D., Taddei, K., Rainey-Smith, S. R., Brown, B. M., Gardener, S. L., Laws, S. M., & Martins, R. N. (2019). Subjective memory complaints predict baseline but not future cognitive function over three years: Results from the Western Australia Memory Study. International Psychogeriatrics, 31(4), 513–525. doi: 10.1017/S1041610218001072 [DOI] [PubMed] [Google Scholar]
- Studer, J., Donati, A., Popp, J., & von Gunten, A. (2014). Subjective cognitive decline in patients with mild cognitive impairment and healthy older adults: Association with personality traits. Geriatrics & Gerontology International, 14(3), 589–595. doi: 10.1111/ggi.12139 [DOI] [PubMed] [Google Scholar]
- Tucker, A. M., & Stern, Y. (2011). Cognitive reserve in aging. Current Alzheimer Research, 8(4), 354–360. doi: 10.2174/156720511795745320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooren, S. A. H., Valentijn, A. M., Bosma, H., Ponds, R. W. H. M., Van Boxtel, M. P. J., & Jolles, J. (2007). Cognitive functioning in healthy older adults aged 64–81: A cohort study into the effects of age, sex, and education. Aging, Neuropsychology, and Cognition, 14(1), 40–54. doi: 10.1080/138255890969483 [DOI] [PubMed] [Google Scholar]
- Villemagne, V. L., Burnham, S., Bourgeat, P., Brown, B., Ellis, K. A., Salvado, O., Szoeke, C., Macaulay, S. L., Martins, R., Maruff, P., Ames, D., Rowe, C. C., & Masters, C. L.; Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group (2013). Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. The Lancet Neurology, 12(4), 357–367. doi: 10.1016/S1474-4422(13)70044-9 [DOI] [PubMed] [Google Scholar]
- Vogel, J. W., Doležalová, M. V., La Joie, R., Marks, S. M., Schwimmer, H. D., Landau, S. M., & Jagust, W. J. (2017). Subjective cognitive decline and β-amyloid burden predict cognitive change in healthy elderly. Neurology, 89(19), 2002–2009. doi: 10.1212/WNL.0000000000004627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfsgruber, S., Kleineidam, L., Guski, J., Polcher, A., Frommann, I., Roeske, S., Spruth, E. J., Franke, C., Priller, J., Kilimann, I., Teipel, S., Buerger, K., Janowitz, D., Laske, C., Buchmann, M., Peters, O., Menne, F., Casan, M. F., Wiltfang, J., … & DELCODE Study Group. (2020). Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology, 95(9), e1134–e1143. doi: 10.1212/WNL.0000000000010142 [DOI] [PubMed] [Google Scholar]
- Yu, H., Wang, K., Zhong, P., Cheng, H. D., Lv, X. Y., & Yuan, L. L. (2020). Investigations of memory monitoring in individuals with subjective cognitive decline and amnestic mild cognitive impairment. Cognitive and Behavioral Neurology, 33(3), 201–207. doi: 10.1097/wnn.0000000000000242 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.