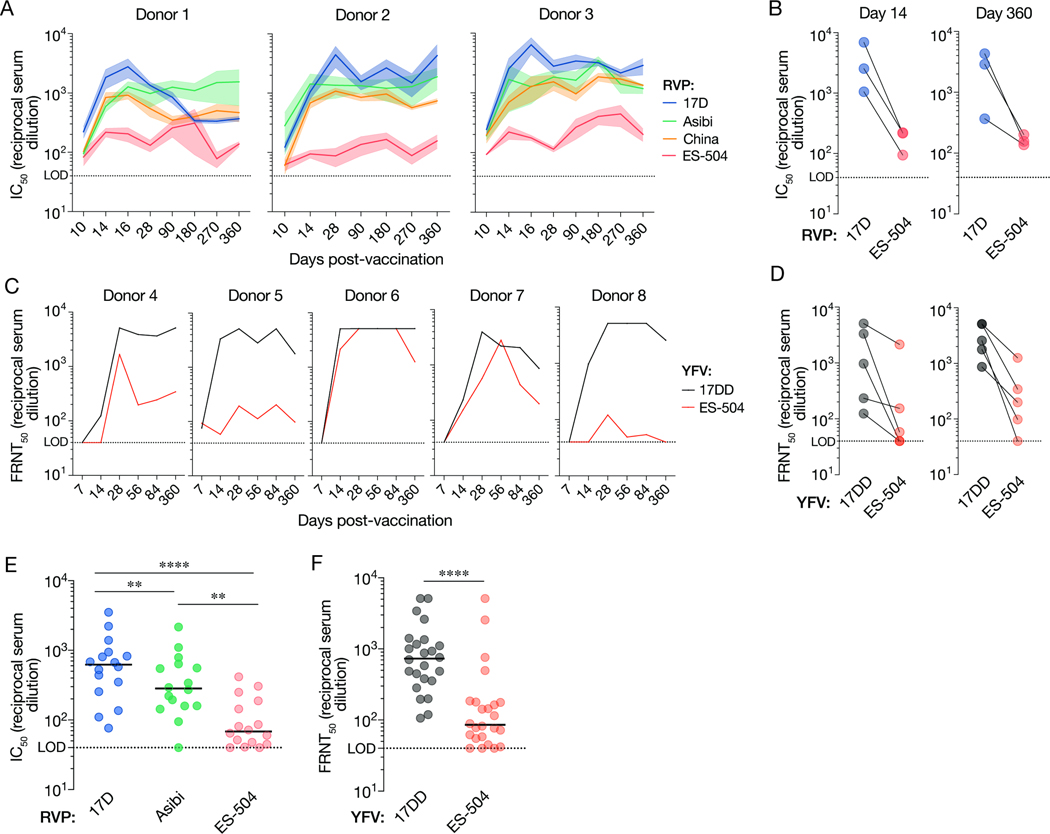
Figure 1. Antiviral potency and breadth of the neutralizing antibody response elicited by YF-17D vaccination.
(A) Serum neutralizing titers (half-maximal inhibitory concentration values from dose-response curves [IC50]) for three YF-17D–vaccinated U.S. donors over time against the indicated RVPs. Means±SEM, n=9–15 from 3–5 independent experiments. (B) Serum neutralizing titers for the three donors in panel A at days 14 and 360 post-vaccination. Means, n=9–15 from 3–5 independent experiments. (C) Serum neutralizing titers (half-maximal inhibitory concentration values in a focus reduction neutralization test [FRNT50]) for five YF-17DD–vaccinated Brazilian donors over time against the indicated authentic viruses. Means, n=3. (D) Serum neutralizing titers (FRNT50) for the five donors in panel C at days 14 and 360 post-vaccination. Means, n=3. (E) Serum neutralizing titers for a U.S. vaccinee cohort (n=16 donors) against the indicated RVPs. n=9–12 from 3–4 independent experiments. (F) Serum neutralizing titers (FRNT50) for a Brazilian vaccinee cohort (n=24 donors against the indicated authentic viruses. n=3. In panels E–F, lines indicate group medians. Groups in E were compared by two-way ANOVA followed by Tukey’s correction for multiple comparisons. Groups in F were compared by the Wilcoxon matched-pairs signed-rank test. **, P<0.002. ****, P<0.0001. LOD, limit of detection.