Abstract
Background
Acute myeloid leukemia with myelodysplasia‐related changes (AML‐MRC) generally confers poor prognosis, however, patient outcomes are heterogeneous. The impact of TP53 allelic state and variant allele frequency (VAF) in AML‐MRC remains poorly defined.
Methods
We retrospectively evaluated 266 AML‐MRC patients who had NGS testing at our institution from 2014 to 2020 and analyzed their clinical outcomes based on clinicopathological features.
Results
TP53 mutations were associated with cytogenetic abnormalities in 5q, 7q, 17p, and complex karyotype. Prognostic evaluation of TP53 MUT AML‐MRC revealed no difference in outcome between TP53 double/multi‐hit state and single‐hit state. Patients with high TP53 MUT variant allele frequency (VAF) had inferior outcomes compared to patients with low TP53 MUT VAF. When compared to TP53 WT patients, TP53 MUT patients had inferior outcomes regardless of MRC‐defining criteria, TP53 allelic state, or VAF. TP53 mutations and elevated serum LDH were independent predictors for inferior OS and EFS, while PHF6 mutations and transplantation were independent predictors for favorable OS and EFS. NRAS mutation was an independent predictor for favorable EFS.
Conclusions
Our study suggests that TP53 MUT AML‐MRC defines a very‐high‐risk subentity of AML in which novel therapies should be explored.
Keywords: AML risk stratification, AML‐MRC, next‐generation sequencing, TP53 mutation
TP53‐mutated AML‐MRC patients have inferior outcomes compared to TP53‐wildtype patients irrespective of MRC‐defining criteria, TP53 allelic state or TP53 variant allele frequency. TP53‐mutated AML‐MRC should be classified as a very‐high‐risk AML subentity for which novel therapeutic strategies should be explored.

1. INTRODUCTION
Acute myeloid leukemia with myelodysplasia‐related changes (AML‐MRC) is a subentity of AML that accounts for approximately 25% of all AML cases. The diagnosis of AML‐MRC requires at least one of the following: (a) a prior history of myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative neoplasm (MDS/MPN), (b) presence of a MDS‐defining cytogenetic abnormality, and (c) morphologic detection of multi‐lineage dysplasia (MLD). AML‐MRC is associated with advanced age, low remission rates, and poor prognosis, with a median overall survival of approximately 12 months. 1 , 2 Nonetheless, AML‐MRC patient outcomes remain heterogeneous. 2 , 3 , 4 , 5
In recent years, next‐generation sequencing (NGS) has become a standard tool to risk stratify patients with myeloid malignancies. In AML, mutations in CEBPA, NPM1, FLT3, RUNX1, ASXL1, and TP53 have shown prognostic relevance and have been incorporated into the 2017 ELN risk stratification. 6 However, there are limited studies that have described the molecular landscape of AML‐MRC and evaluated the prognostic impact of mutations within this subentity.
TP53, which is located on the short arm of chromosome 17, encodes the tumor suppressor protein p53 that mediates critical anti‐tumor activity by inducing apoptosis in response to DNA damage. 7 TP53 mutations are detected in up to 10% of de novo AML patients, however, its incidence has been shown to increase to approximately 30% in AML‐MRC. 8 , 9 , 10 TP53 mutations and abnormalities in chromosome 17p have been identified as poor prognostic factors by the 2017 ELN risk stratification and are associated with advanced age, therapy resistance, and poor prognosis. 6 , 11 , 12 TP53 mutations were recently identified to have an adverse prognostic impact in AML‐MRC. 5 However, it remains unclear whether TP53 allelic state and variant allele frequency (VAF) can further resolve the heterogeneity in AML‐MRC patient outcomes.
In the present study, we performed an extensive molecular evaluation of 266 AML‐MRC patients and retrospectively assessed whether individual mutations were associated with patient outcomes. We further characterized TP53‐mutated (TP53 MUT) AML‐MRC to evaluate whether TP53 allelic state and clonal burden could resolve prognostic heterogeneity in AML‐MRC.
2. MATERIALS AND METHODS
2.1. Patients and therapy
We screened all the University Health Network patients diagnosed with AML‐MRC as per the 2016 WHO definition between April 2014 and November 2020. The study was approved by the University Health Network Research Ethics Board. Peripheral blood and bone marrow aspirate smears were reviewed by at least two independent hematopathologists and consensus on diagnosis was achieved. Patients were classified into three groups: (a) AML‐MRC patients with a history of MDS or of MDS/MPN, irrespective of the presence of MDS‐related cytogenetic changes (AML‐MRC‐H); (b) AML‐MRC patients with MDS‐defining cytogenetic abnormalities (AML‐MRC‐C); and (c) AML‐MRC patients with MLD alone, defined as the presence of >50% dysplasia in at least two lineages (AML‐MRC‐M). Patients with therapy‐related AML were excluded from this study. The majority of patients were treated with induction regimen consisting of cytarabine and daunorubicin/idarubicin, or non‐intensive regimens consisting of hypomethylating agent (HMA) or low‐dose cytarabine.
2.2. Karyotype analysis
In accordance with the International System for Human Cytogenetic Nomenclature guidelines, karyotypes were obtained from diagnostic bone marrow samples and described as appropriate. The cytogenetic loss of TP53 was determined as previously described. 13 Complex karyotype was defined as the presence of at least three cytogenetic abnormalities.
2.3. Mutational analysis and allocation of patients based on TP53 allelic status
Next‐generation sequencing (NGS) was performed for 105 patients using a custom myeloid panel for 49 genes implicated in myeloid malignancies (Oxford Gene Technologies) and run on the MiSeq platform (Illumina), as previously described. 14 , 15 For 161 patients, NGS was performed using the TruSight Myeloid Sequencing Panel for 54 genes implicated in myeloid malignancies (Illumina) and run on the MiSeq platform (Illumina), as previously described. 16 , 17 The limit of detection for variant calling was 2%. On both panels, for 13/41 genes, the complete coding regions were sequenced, and for 28/41 genes, the same exonic hotspots were sequenced (Tables S1 and S2). Interpretation and classification of variants were performed as previously described. 15 Variants detected by NGS are listed in Table S3. Of note, TP53 mutations were assessed by NGS which spanned TP53 exons 2–11 (Table S2), variants of unknown significance were excluded from analysis. When patients had multiple mutations in the same gene, the higher VAF was used for analysis.
Patients were considered to be double/multi‐hit TP53 state when (A) at least two TP53 variants were detected by NGS, (B) one TP53 variant detected by NGS co‐occurred with cytogenetic loss of TP53 and (C) one TP53 variant was detected by NGS with a VAF of ≥55%, as previously described. 18
2.4. Statistical analysis
Evaluation of patient outcomes including overall survival (OS) and event‐free survival (EFS) was carried out from retrospective analysis of patient records. OS was calculated as the time from the date of diagnosis to last follow‐up or death. EFS was defined as the time from the date of diagnosis to last follow‐up, relapse, or death. Univariate survival analysis and comparison of outcome were performed using the Kaplan–Meier product‐limit method and the log‐rank test. The chi‐square or Fisher's exact test, as appropriate, was used to assess associations between categorical parameters. The Kruskal–Wallis and Wilcoxon rank‐sum tests were used to assess whether there were differences in numerical variables between groups, as appropriate. Multivariable Cox proportional hazards regression was performed with variables that were significant prognostic factors by univariable analysis and variables that are known prognostic factors in AML. All statistical tests were performed using R software version 4.0.5 (R Core Team [2020]. R: A language and environment for statistical computing, Vienna, Austria) and were interpreted as significant if the two‐sided p‐value was less than 0.05.
3. RESULTS
3.1. Patient characteristics by MRC subtype and TP53 mutation status
Two hundred and sixty‐six AML‐MRC patients were identified, including 142 (53.4%) AML‐MRC‐C patients, 99 (37.2%) AML‐MRC‐H patients, and 25 (9.4%) AML‐MRC‐M patients. The median age of our cohort was 70 years (range 18–91). The median follow‐up time was 6.2 months (range 0–75.7 months) and 160 (60%) patients died at the time of last follow‐up.
Patient characteristics as stratified by AML‐MRC subtype are summarized and compared in Table 1. Age, gender ratio, WBC count, platelet count, hemoglobin, serum LDH, and transplantation status were not significantly different across the three subtypes. Bone marrow blast % was elevated in AML‐MRC‐C compared to AML‐MRC‐H and AML‐MRC‐M (p = 0.032 and p = 0.018, respectively). The number of mutated genes was higher in AML‐MRC‐H and AML‐MRC‐M compared to AML‐MRC‐C (p = 0.00015 and p < 0.0001, respectively).
TABLE 1.
Comparison of clinical features between AML‐MRC‐C, AML‐MRC‐H, and AML‐MRC‐M
| Clinical feature | Total (n = 266) | AML‐MRC‐C (n = 142) | AML‐MRC‐H (n = 99) | AML‐MRC‐M (n = 25) | p‐value |
|---|---|---|---|---|---|
| Age (y), median [range] | 70 [18–91] | 70 [30–91] | 70 [19–89] | 73 [18–90] | 0.475 a |
| Male, n (%) | 168 (63) | 86 (61) | 64 (65) | 18 (72) | 0.510 b |
| WBC count ×109/L, median [range] | 3.4 [0.1–328.7] | 3.4 [0.3–292.4] | 3.7 [0.1–328.7] | 3.2 [0.8–60.6] | 0.606 a |
| Platelets ×109/L, median [range] | 47 [3–1057] | 47.5 [7–1057] | 42 [3–703] | 52.5 [14–490] | 0.602 a |
| Hemoglobin, g/dL, median [range] | 85 [8–671] | 85 [8–671] | 87 [57–148] | 82 [55–108] | 0.452 a |
| BM blasts %, median [range] | 39.5 [20–95] | 48 [20–95] | 35 [20–91] | 31 [20–82] | 0.017 a |
| LDH, IU/L, median [range] | 304 [90–9473] | 304 [94–9473] | 305 [101–6550] | 277 [90–1566] | 0.535 a |
| Number of mutated genes, median [range] | 3 [0–9] | 2 [0–8] | 3 [0–9] | 4 [1–7] | <0.0001 a |
| Allo‐HSCT, n (%) | 57 (21) | 30 (21) | 23 (23) | 4 (16) | 0.727 b |
Abbreviations: Allo‐HSCT, allogeneic hematopoietic stem cell transplantation; BM, bone marrow; LDH, lactate dehydrogenase; WBC, white blood cell.
Kruskal–Wallis test.
Chi‐square test.
Patient characteristics as stratified by TP53 mutation status are summarized and compared in Table 2. Age, WBC count, platelet count, hemoglobin, bone marrow blast %, and serum LDH were not statistically different between TP53 MUT and TP53 WT patients. However, the TP53 MUT group had a greater female representation and had fewer mutated genes other than TP53 compared to the TP53 WT group. Fewer TP53 MUT patients had bone marrow transplantation.
TABLE 2.
Comparison of clinical features between TP53 MUT and TP53 WT AML‐MRC
| Clinical feature | Total (n = 266) | TP53 MUT (n = 96) | TP53 WT (n = 170) | p‐value |
|---|---|---|---|---|
| Age (y), median [range] | 70 [18–91] | 71.5 [38–91] | 70 [18–90] | 0.11 a |
| Male, n (%) | 168 (63) | 51 (53) | 117 (69) | 0.016 b |
| WBC count ×109/L, median [range] | 3.4 [0.1–328.7] | 3.1 [0.1–76.9] | 3.7 [0.5–328.7] | 0.14 a |
| Platelets ×109/L, median [range] | 47 [3–1057] | 46 [3–355] | 48 [7–1057] | 0.078 a |
| Hemoglobin, g/dL, median [range] | 85 [8–671] | 84 [57–671] | 86 [8–169] | 0.445 a |
| BM blasts %, median [range] | 39.5 [20–95] | 38 [20–91] | 40 [20–95] | 0.493 a |
| LDH, IU/L, median [range] | 304 [90–9473] | 320 [134–3569] | 294 [90–9473] | 0.11 a |
| Number of non‐TP53 mutated genes, median [range] | 2 [0–9] | 1 [0–4] | 3 [0–9] | <0.0001 a |
| Allo‐HSCT, n (%) | 57 (21) | 12 (13) | 45 (26) | 0.012 b |
Abbreviations: Allo‐HSCT, allogeneic hematopoietic stem cell transplantation; BM, bone marrow; LDH, lactate dehydrogenase; WBC, white blood cell.
Wilcoxon rank‐sum test.
Chi‐square test.
3.2. Mutation landscape of AML‐MRC
The mutational landscape of AML‐MRC is presented in Figure 1. The most frequently mutated gene was TP53, mutated in 96 (36%) patients, followed by DNMT3A (26%), ASXL1 (21%), TET2 (21%), RUNX1 (19%), SRSF2 (15%), IDH2 (14%), U2AF1 (11%), and STAG2 (11%). 140 (53%) patients had at least one secondary‐type mutation involving ASXL1, BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1, and ZRSR2, as defined by Lindsley et al. 19
FIGURE 1.
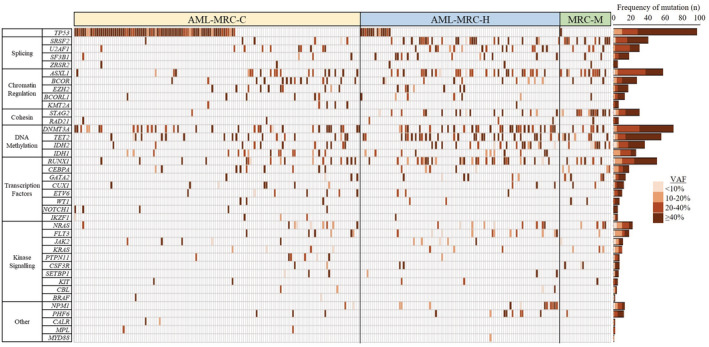
Co‐mutation plot for AML‐MRC patients. Mutations are colored by their variant allele frequency.
The frequency of mutations in each AML‐MRC subtype is presented in Figure S1 and an association analysis of the frequently mutated genes is described here. TP53 mutations were significantly enriched in AML‐MRC‐C compared to both AML‐MRC‐H and AML‐MRC‐M (p < 0.0001 and p < 0.0001, respectively). STAG2 mutations were significantly enriched in AML‐MRC‐M compared to both AML‐MRC‐C and AML‐MRC‐H (p < 0.0001 and p = 0.0004, respectively), and were significantly enriched in AML‐MRC‐H compared to AML‐MRC‐C (p < 0.0001). Mutations significantly enriched in both AML‐MRC‐H and AML‐MRC‐M relative to AML‐MRC‐C were in ASXL1 (p = 0.011 and p = 0.0008, respectively), SRSF2 (p < 0.0001 and p < 0.0001, respectively) and IDH2 (p = 0.041 and p = 0.0021, respectively). Mutations in U2AF1, RUNX1 and TET2 were significantly enriched only in AML‐MRC‐H compared to AML‐MRC‐C (p = 0.042, p = 0.0043 and p = 0.0040, respectively). Overall, AML‐MRC‐C was characterized by TP53 mutations, while AML‐MRC‐H and AML‐MRC‐M were characterized by secondary‐type mutations. Indeed, secondary‐type mutations were more frequently harbored in AML‐MRC‐M (21/25 patients, 84%) and AML‐MRC‐H (67/99 patients, 68%) compared to AML‐MRC‐C (52/142 patients, 37%) (p < 0.0001 and p < 0.0001, respectively).
3.3. Mutational complementation groups in AML‐MRC
Molecular characterization of AML‐MRC allowed us to identify frequently co‐occurring and mutually exclusive mutations in AML‐MRC. Our correlation analysis of recurrently (≥5) mutated genes is presented in Figure S2. Mutations in ASXL1, RUNX1, SRSF2, and IDH2 were significantly underrepresented in the TP53 MUT group. ASXL1 mutations significantly co‐occurred with mutations in RUNX1, SRSF2, IDH2, and STAG2. STAG2 mutations also significantly co‐occurred with mutations in SRSF2, IDH2, and NRAS. Other significantly co‐occurring mutation pairs included IDH2 and SRSF2, IDH2 and FLT3, and U2AF1 and KRAS.
3.4. Association of TP53 mutations with cytogenetic abnormalities
Karyotype analysis was available in 248 (93%) AML‐MRC patients, including 92 TP53 MUT and 156 TP53 WT patients. TP53 MUT patients were more likely to have complex karyotype than TP53 WT patients (89/92 patients, 97% vs. 41/156 patients, 26%, respectively, p < 0.0001). TP53 MUT patients were also more likely to have cytogenetic abnormalities in chromosome 17p (41/92 patients, 45% vs. 7/156 patients, 5%, respectively, p < 0.0001).
Among the 18 patients without available karyotype, 12 patients had interphase FISH for del(5q) and del(7q). TP53 MUT patients were more likely to have chromosomal abnormalities in 5q (71/95 patients, 75% vs. 23/165 patients, 14%, respectively, p < 0.0001) and 7q (54/95 patients, 57% vs. 38/165, 23%, respectively, p < 0.0001).
Overall, chromosomal aberrations in 17p had the highest specificity (95%) for TP53 mutations, and complex karyotype had the highest sensitivity (97%) for TP53 mutations.
3.5. Outcomes of AML‐MRC patients based on subtype and genetic features
The median OS and EFS of our cohort were 10.7 months and 7.7 months, respectively. Kaplan–Meier curves for patients as stratified by subtype are presented in Figure S3A,B. AML‐MRC‐M patients had borderline significantly better OS (HR 0.59, 95% CI 0.34–1.02, p = 0.058) and had significantly better EFS (HR 0.58, 95% CI 0.35–0.97, p = 0.037) compared to AML‐MRC‐C patients. AML‐MRC‐H patients had borderline significantly better OS (HR 0.74, 95% CI 0.53–1.04, p = 0.081) and EFS (HR 0.75, 95% CI 0.54–1.02, p = 0.068) compared to AML‐MRC‐C patients. AML‐MRC‐M patients had no significant differences in OS (HR 0.78, 95% CI 0.44–1.39, p = 0.40) and EFS (HR 0.77, 95% CI 0.45‐1.32, p = 0.34) compared to AML‐MRC‐H patients.
Given the paucity of data on the prognostic impact of mutations in AML‐MRC, we evaluated the associations between individual gene mutations and patient outcomes. The OS and EFS hazard ratios, median and 2‐year OS and EFS, and log‐rank p‐values for all mutations are presented in Figure 2. TP53 mutations were associated with inferior OS and EFS, while mutations in IDH1, NRAS, and PHF6 were associated with favorable OS and EFS. SF3B1 mutations were associated with favorable OS but not EFS. The respective Kaplan–Meier curves are provided in Figures S4 and S5.
FIGURE 2.

Forest plot indicating the OS and EFS hazard ratio and 95% confidence interval of mutations in AML‐MRC. Median and 2‐year survival and log‐rank p‐value are listed to the right.
Kaplan–Meier curves for patients as stratified by both subtype and TP53 mutation status are presented in Figure 3A,B. TP53 mutations were detected in 80 (56%) AML‐MRC‐C patients, 15 (15%) AML‐MRC‐H patients, and one (4%) AML‐MRC‐M patient. TP53 mutation had an adverse impact on OS and EFS in AML‐MRC‐C (HR 2.52, 95% CI 1.62–3.93, p < 0.0001 and HR 2.14, 95% CI 1.42–3.21, p = 0.0002, respectively). TP53 mutation was associated with borderline significantly inferior OS (HR 1.93, 95% CI 0.98–3.78, p = 0.053) and significantly inferior EFS (HR 1.89, 95% CI 0.99–3.59, p = 0.049, respectively) in AML‐MRC‐H. For both TP53 MUT and TP53 WT patients, AML‐MRC subtype had no significant impact on OS (TP53 MUT: p = 0.39; TP53 WT: p = 0.79) or EFS (TP53 MUT: p = 0.43; TP53 WT: p = 0.68).
FIGURE 3.

Kaplan–Meier estimates for OS and EFS of AML‐MRC patients stratified by (A, B) AML‐MRC subtype and TP53 mutation status, (C, D) number of hits to TP53, (E, F) median TP53 variant allele frequency (VAF).
Cytogenetic loss of TP53 was associated with inferior OS (HR 2.49, 95% CI 1.70–3.63, p < 0.0001) and EFS (HR 2.25, 95% CI 1.57–3.23, p < 0.0001) (Figure S6A,B). Among patients with TP53 MUT, additional TP53 variant(s) were detected in 26 patients and did not significantly impact OS (HR 0.68, 95% CI 0.39–1.17, p = 0.16) or EFS (HR 0.65, 95% CI 0.38–1.11, p = 0.11) (Figure S6C,D). Compared to TP53 WT patients, TP53 MUT patients with one TP53 mutation had inferior OS (HR 2.79, 95% CI 1.96–3.96, p < 0.0001) and EFS (HR 2.56, 95% CI 1.83–3.57, p < 0.0001), and TP53 MUT patients with multiple TP53 mutations had inferior OS (HR 1.89, 95% CI 1.12–3.19, p = 0.015) and borderline significantly inferior EFS (HR 1.63, 95% CI 0.99–2.69, p = 0.054) (Figure S6C,D). Among patients with TP53 MUT, concurrent cytogenetic loss of TP53 did not significantly impact OS (HR 1.35, 95% CI 0.83–2.20, p = 0.22) or EFS (HR 1.31, 95% CI 0.82–2.10, p = 0.25) (Figure S6E,F). Compared to unaltered TP53 patients, all altered TP53 patients, either with cytogenetic loss of TP53 alone, TP53 mutation alone, or TP53 mutation with concurrent cytogenetic loss of TP53 had inferior OS (HR 2.90, 95% CI 1.32–6.36, p = 0.0055; HR 2.36, 95% CI 1.58–3.52, p < 0.0001; HR 3.14, 95% CI 2.03–4.84, p < 0.0001, respectively) and EFS (HR 2.34, 95% CI 1.08–5.10, p = 0.027; HR 2.07, 95% CI 1.42–3.04, p < 0.0001; HR 2.81, 95% CI 1.86–4.24, p < 0.0001, respectively) (Figure S6E,F).
A workflow to allocate TP53 allelic state in our cohort is presented in Figure S7. AML‐MRC patients with TP53 double/multi‐hit had no significant differences in OS (HR 1.10, 95% CI 0.60–2.01, p = 0.76) and EFS (HR 1.20, 95% CI 0.66–2.19, p = 0.55) compared to patients with TP53 single‐hit (Figure 3C,D). Compared to unaltered TP53 patients, both TP53 single‐hit and TP53 double/multi‐hit patients had inferior OS (HR 2.48, 95% CI 1.35–4.54, p = 0.0023 and HR 2.73, 95% CI 1.93–3.85, p < 0.0001, respectively) and EFS (HR 2.08, 95% CI 1.15–3.76, p = 0.014 and HR 2.40, 95% CI 1.73–3.31, p < 0.0001, respectively) (Figure 3C,D).
When stratified by the median TP53 MUT VAF (52.1%), patients with high VAF had inferior OS (HR 1.75, 95% CI 1.09–2.82, p = 0.019) and EFS (HR 1.90, 95% CI 1.18–3.05, p = 0.0068) compared to low VAF patients (Figure 3E,F). Compared to TP53 WT patients, all TP53 MUT patients with either low or high TP53 MUT VAF had inferior OS (HR 1.93, 95% CI 1.29–2.89, p = 0.0012 and HR 3.38, 95% CI 2.26–5.05, p < 0.0001, respectively) and EFS (HR 1.67, 95% CI 1.13–2.47, p = 0.0089 and HR 3.20, 95% CI 2.19–4.68, p < 0.0001, respectively) (Figure 3E,F). Similar results were observed with TP53 MUT VAF threshold of 40%: AML‐MRC patients with high VAF had borderline significantly inferior OS (HR 1.59, 95% CI 0.93–2.73, p = 0.086) and EFS (HR 1.67, 95% CI 0.99–2.82, p = 0.053) compared to low VAF patients (Figure S8A,B). Compared to TP53 WT patients, all AML‐MRC patients with either TP53 MUT VAF <40% or ≥40% had inferior OS (HR 1.84, 95% CI 1.10–3.08, p = 0.018 and HR 2.81, 95% CI 1.98–4.00, p < 0.0001, respectively) and EFS (HR 1.63, 95% CI 1.00–2.67, p = 0.050 and HR 2.56, 95% CI 1.83–3.57, p < 0.0001, respectively) (Figure S8A,B).
In TP53 MUT AML‐MRC patients, concurrent DNMT3A mutation was associated with inferior OS (HR 2.12, 95% CI 1.20–3.76, p = 0.0083) and EFS (HR 1.96, 95% CI 1.11–3.45, p = 0.018), while in TP53 WT patients, DNMT3A mutation did not significantly impact OS (HR 0.81, 95% CI 0.51–1.30, p = 0.39) or EFS (HR 0.84, 95% CI 0.55–1.28, p = 0.41) (Figure S9A,B).
Interestingly, AML‐MRC patients with ≥6 mutated genes had better OS (HR 0.46, 95% CI 0.23–0.90, p = 0.02) and EFS (HR 0.50, 95% CI 0.27–0.92, p = 0.024) compared to patients with <6 mutated genes (Figure S10A,B). Similarly, when using a threshold of three mutations, patients with higher number of mutated genes had borderline significantly better OS (HR 0.74, 95% CI 0.54–1.01, p = 0.055) and EFS (HR 0.78, 95% CI 0.58–1.05, p = 0.097) (Figure S10C,D).
Both TP53 WT and TP53 MUT patients who went onto transplant had significantly longer OS (HR 0.13, 95% CI 0.06–0.28, p < 0.0001 and HR 0.37, 95% CI 0.17–0.77, p = 0.0063, respectively) and EFS (HR 0.31, 95% CI 0.19–0.52, p < 0.0001 and HR 0.44, 95% CI 0.22–0.87, p = 0.015, respectively) than those who did not, however, TP53 WT patients had dramatically improved and plateauing long‐term survival rates (Figure S11A–D).
By multivariable analysis for OS, TP53 mutation and elevated serum LDH levels were independent predictors for adverse outcomes, while PHF6 mutation and transplantation were independently associated with improved outcomes (Table 3). For EFS, TP53 mutation and elevated serum LDH levels were independent predictors for adverse outcomes, while NRAS mutation, PHF6 mutation, and transplantation were independently associated with improved outcomes (Table 3).
TABLE 3.
Multivariable analysis for overall survival and event‐free survival
| Characteristic | Overall survival | Event free survival | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | p‐value | |
| TP53 mutation | 1.77 (1.17–2.67) | 0.007 | 1.60 (1.10–2.33) | 0.013 |
| SF3B1 mutation | 0.50 (0.23–1.09) | 0.082 | — | — |
| IDH1 mutation | 0.81 (0.42–1.58) | 0.539 | 0.69 (0.38–1.27) | 0.239 |
| PHF6 mutation | 0.15 (0.03–0.87) | 0.034 | 0.15 (0.03–0.78) | 0.024 |
| NRAS mutation | 0.58 (0.28–1.19) | 0.136 | 0.49 (0.25–0.96) | 0.039 |
| Age a | 1.00 (0.99–1.02) | 0.865 | 1.00 (0.99–1.01) | 0.876 |
| WBC count a | 1.00 (0.99–1.00) | 0.936 | 1.00 (1.00–1.00) | 0.971 |
| LDH a | 1.00 (1.00–1.00) | 0.0010 | 1.00 (1.00–1.00) | 0.0013 |
| AML‐MRC subtype | ||||
| AML‐MRC‐M | Reference | — | — | — |
| AML‐MRC‐C | 1.38 (0.74–2.59) | 0.312 | 1.48 (0.83–2.63) | 0.182 |
| AML‐MRC‐H | 1.37 (0.76–2.45) | 0.295 | 1.35 (0.78–2.33) | 0.283 |
| Allo‐HSCT | 0.21 (0.12–0.37) | <0.0001 | 0.37 (0.24–0.58) | <0.0001 |
Abbreviations: Allo‐HSCT, allogeneic hematopoietic stem cell transplantation; LDH, lactate dehydrogenase; WBC, white blood cell.
Age, WBC count, and LDH were analyzed as continuous variables.
4. DISCUSSION
In this study, we report, to the best of our knowledge, the largest molecular profiling of AML‐MRC by detailed NGS. We provide a comprehensive evaluation of the prognostic impact of commonly occurring myeloid gene mutations in AML‐MRC. We identified TP53, PHF6, and NRAS mutations to be independent predictors of outcome. In addition, we provide an in‐depth characterization and prognostic evaluation of TP53 MUT AML‐MRC, revealing that (A) the overall adverse prognosis in AML‐MRC is largely driven by TP53 mutation status and not AML‐MRC‐defining criteria, and (B) TP53 mutation confers an adverse prognostic impact in AML‐MRC irrespective of its allelic state or VAF.
Recent advances in molecular analysis has greatly improved AML risk stratification and classification. The 2022 WHO classification of AML has incorporated genomic alterations in NPM1, CEBPA, and secondary‐type mutations in ASXL1, BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1, and ZRSR2 owing to their association with distinct clinical features and outcomes. 20 However, the 2022 WHO classification still relies heavily on cytogenetic alterations to define AML subentities and does not consider TP53 mutations to define a standalone AML subtype. 20 In contrast, the new 2022 International Consensus Classification of Myeloid Neoplasms and Acute Leukemias recognizes “AML with mutated TP53” as a distinct disease category owing to its association with distinctly aggressive disease, complex cytogenetic abnormalities and very poor outcome. 21 In our cohort, TP53 mutations were independently associated with adverse outcomes in AML‐MRC. Strikingly, when stratifying patients by both AML‐MRC subtype and TP53 mutation status, patient outcomes largely grouped together by TP53 mutation status, not AML‐MRC subtype (Figure 3A,B). This suggests that it is the underlying biology of TP53 mutations rather than AML‐MRC criteria such as MDS‐defining cytogenetic abnormalities that confer adverse outcomes in AML. With the emergence of new therapeutic agents that target specific genetic alterations, there is a strong rationale for a genomics‐based AML classification system.
To date, frontline therapy for AML‐MRC has remained unaltered over the past 40+ years as induction with cytarabine and daunorubicin, followed by transplantation in complete remission. The recently approved CPX‐351 liposomal formulation of cytarabine and daunorubicin was shown to significantly improve secondary AML patient outcomes compared to conventional 7 + 3. 22 , 23 , 24 However, TP53 MUT AML patients treated with CPX‐351 or other chemotherapy regimens have adverse outcomes. 25 , 26 , 27 , 28 , 29 , 30 In addition, studies have shown that TP53 MUT patients who go on to transplant have dismal long‐term outcomes, which is consistent with our data that show TP53 WT patients and not TP53 MUT patients had dramatically improved outcomes and plateauing long‐term survival rates after transplantation. 31 , 32 While the treatment of TP53 MUT remains a challenge, clinical trials for TP53 MUT AML using targeted therapies and immunotherapies have shown great potential. 33 , 34 , 35 , 36 , 37 , 38 , 39
With the emergence of clinical trials for TP53 MUT AML patients, there is an increasing need to timely detect TP53 mutations at the start of treatment. The introduction of NGS covering the prognostically relevant 2017 ELN mutations has largely negated the necessity of more specific molecular tests such as PCR or FISH. To date, TP53 mutation is commonly assessed only by NGS, including at our institution. However, with the slow turnaround times for NGS, physicians might not be able to identify TP53 MUT AML patients at the start of treatment, thus leading to suboptimal therapeutic decisions. Therefore, faster turnaround tests for TP53 mutation should be further explored in the clinical setting and implemented into the standard AML patient workup. However, as we show here, chromosomal aberrations in 5q, 7q, 17p, and complex karyotype may serve as surrogate markers for TP53 mutations.
The prognostic impact of TP53 allelic state in AML remains controversial. As a tumor suppressor gene, it is conventionally thought that TP53 alterations should have a pathogenic effect only in the bi‐allelic or “double‐hit” setting. In MDS, bi‐allelic and not mono‐allelic TP53 alterations have been shown to predict for distinctly adverse outcomes and poor response to therapy, 40 , 41 thus “MDS with bi‐allelic TP53 alteration” has been proposed as a provisional entity in the upcoming 5th edition of the WHO classification of myeloid neoplasms and acute leukemia. 42 In AML, the prognostic impact of TP53 allelic state remains controversial and studies have reported conflicting data. 5 , 41 , 43 , 44 Interestingly, molecular analysis of serial patient samples has provided evidence that AML therapy induces evolutionary pressures for loss‐of‐heterozygosity and expansion of newly acquired double‐hit TP53 cell populations 45 , 46 Thus, AML patients initially presenting with mono‐allelic TP53 alteration may have similar clinical outcomes as patients with bi‐allelic TP53 alteration. Here, we report that TP53 allelic state does not have prognostic implication in AML‐MRC. No significant difference in outcome was observed in AML‐MRC patients when (A) stratifying patients by number of TP53 mutations, (B) stratifying TP53 MUT patients by the presence/absence of cytogenetic loss of TP53, or (C) stratifying patients by the number of TP53 hits. Our data suggest that AML‐MRC with any number of TP53 hits should be considered as very‐high‐risk AML.
The association between TP53 clonal burden and AML patient outcomes remains controversial. Prior studies have reported conflicting data on the prognostic impact of TP53 MUT VAF in both non‐intensive and induction chemotherapy patients. 18 , 43 , 47 , 48 , 49 Here, we report that AML‐MRC patients with high TP53 MUT VAF had inferior OS and EFS compared to patients with low VAF. Therefore, AML‐MRC patients with high TP53 MUT VAF may benefit most from novel TP53 MUT therapies. However, low TP53 MUT VAF was also associated with poor outcomes and should still be considered as a high‐risk factor for AML‐MRC.
It is conventionally thought that greater mutational complexity predicts for inferior outcomes due to clonal heterogeneity. Prior studies have reported associations between higher number of mutations and inferior outcomes in AML. 8 , 50 Interestingly, we showed that in AML‐MRC, higher number of mutated genes was associated with favorable outcomes. TP53 mutations, which independently predicted for poor outcomes in AML‐MRC, were infrequently accompanied by co‐occurring mutations, which was consistent with a prior report. 51 As such, the discrepancy in the prognostic impact of mutational complexity in AML overall and AML‐MRC may be partly explained by the higher frequency of TP53 mutations in AML‐MRC.
In addition to identifying TP53 mutations as having independent prognostic value, we also showed that NRAS and PHF6 mutations were independent predictors for improved outcomes. Even after exclusion of AML‐MRC‐M cases, PHF6 mutations retained their association with improved OS (HR 0.06, 95% CI 0.006–0.69, p = 0.024) and EFS (HR 0.06, 95% CI 0.006–0.65, p = 0.020), while NRAS mutations retained their association with improved EFS (HR 0.44, 95% CI 0.21–0.91, p = 0.028) in multivariable analysis (data not shown). NRAS mutations were reported to have a favorable prognostic effect in NPM1 MUT/DNMT3A MUT AML. 8 While NRAS mutations have not shown a significant prognostic impact in AML overall, studies have shown that NRAS‐mutated clones are chemosensitive and do not persist after treatment. 52 , 53 , 54 , 55 , 56 In contrast, there are limited data on the prognostic impact of PHF6 mutations in AML, likely due to its low frequency (2%–3%) in AML. 57 Patel et al 58 reported that PHF6 mutations were associated with adverse outcomes in AML overall and intermediate‐risk AML. Another study in CK‐AML patients revealed that when stratifying patients into “typical CK”, defined as CK containing high‐risk abnormalities in 5q, 7q, and/or 17p, and “atypical CK”, defined as CK without such abnormalities, CK‐AML patients with atypical CK had a significantly higher rate of PHF6 mutation and favorable outcomes. 59 Here, we show for the first time that NRAS and PHF6 mutations have an independently favorable prognostic impact in AML‐MRC. Future studies are needed to evaluate whether NRAS MUT or PHF6 MUT AML‐MRC should still be classified as high‐risk AML.
We acknowledge that our study has several limitations. First, our study is limited by its retrospective nature and its relatively small subsets for analysis. Future prospective studies with larger cohorts are needed to confirm our data. Second, NGS was performed with two different gene panels which may have resulted in variability in the reported VAFs due to technical differences. However, all NGS experiments were performed on the same MiSeq platform. Third, our TP53 MUT VAF threshold of the median may not reflect a biologically meaningful threshold, however, this also applies to other prognostic biomarkers such as WBC count. Future investigations are needed to optimize the TP53 MUT VAF threshold for risk stratification.
5. CONCLUSION
In conclusion, TP53 MUT AML‐MRC patients had inferior outcomes compared to TP53 WT patients irrespective of MRC‐defining criteria, TP53 allelic state, or TP53 VAF. Our study suggests that TP53 MUT AML‐MRC should be classified as a very‐high‐risk AML subentity and highlights the great need for novel therapies for TP53 MUT AML.
AUTHORS’ CONTRIBUTIONS
DZ analyzed data, performed statistical analysis, and wrote the manuscript. DZ, EE, MZ, JM, AS and MM collected data for analysis. EA performed statistical analysis. HC designed the study, analyzed data, supervised the project, and wrote the manuscript.
FUNDING INFORMATION
This research was supported by grants from the Leukemia and Lymphoma Research Society of Canada (LLSC) and the Cancer Research Society (CRS).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS APPROVAL STATEMENT
The study was approved by the UHN Research Ethics Board.
Supporting information
Data S1
ACKNOWLEDGMENTS
This research was supported by grants from the Leukemia and Lymphoma Research Society of Canada (LLSC) and the Cancer Research Society (CRS).
Zhao D, Eladl E, Zarif M, et al. Molecular characterization of AML‐MRC reveals TP53 mutation as an adverse prognostic factor irrespective of MRC‐defining criteria, TP53 allelic state, or TP53 variant allele frequency. Cancer Med. 2023;12:6511‐6522. doi: 10.1002/cam4.5421
DATA AVAILABILITY STATEMENT
Data are available on request from the corresponding author.
REFERENCES
- 1. Koenig KL, Sahasrabudhe KD, Sigmund AM, Bhatnagar B. AML with myelodysplasia‐related changes: development, challenges, and treatment advances. Genes (Basel). 2020;11(8):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arber DA, Erba HP. Diagnosis and treatment of patients with acute myeloid leukemia with myelodysplasia‐related changes (AML‐MRC). Am J Clin Pathol. 2020;154(6):731‐741. Available from:. https://academic.oup.com/ajcp/article/154/6/731/5899448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Díaz‐Beyá M, Rozman M, Pratcorona M, et al. The prognostic value of multilineage dysplasia in de novo acute myeloid leukemia patients with intermediate‐risk cytogenetics is dependent on NPM1 mutational status. Blood. 2010;116(26):6147‐6148. Available from:. http://ashpublications.org/blood/article‐pdf/116/26/6147/1491587/zh805210006147.pdf [DOI] [PubMed] [Google Scholar]
- 4. Miesner M, Haferlach C, Bacher U, et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS‐related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: a comparison of 408 cases classified as “AML not otherwise specified” (AML‐NOS) or “AML with myelodysplasia‐related changes” (AML‐MRC). Blood. 2010;116(15):2742‐2751. [DOI] [PubMed] [Google Scholar]
- 5. Montalban‐Bravo G, Kanagal‐Shamanna R, Class CA, et al. Outcomes of acute myeloid leukemia with myelodysplasia related changes depend on diagnostic criteria and therapy. Am J Hematol. 2020;95(6):612‐622. [DOI] [PubMed] [Google Scholar]
- 6. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A. How does p53 induce apoptosis and how does this relate to p53‐mediated tumour suppression? Cell Death Differ. 2018;25(1):104‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209‐2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cancer Genome Atlas Research Network , Ley TJ, Miller C, et al. Genomic and epigenomic landscapes of adult De novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059‐2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badar T, Szabo A, Sallman D, et al. Interrogation of molecular profiles can help in differentiating between MDS and AML with MDS‐related changes. Leuk Lymphoma. 2020;61(6):1418‐1427. [DOI] [PubMed] [Google Scholar]
- 11. Molica M, Mazzone C, Niscola P, de Fabritiis P. TP53 mutations in acute myeloid leukemia: still a daunting challenge? Front Oncol. 2021;10:610820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barbosa K, Li S, Adams PD, Deshpande AJ. The role of TP53 in acute myeloid leukemia: challenges and opportunities. Genes Chromosomes Cancer. 2019;58(12):875‐888. [DOI] [PubMed] [Google Scholar]
- 13. Montalban‐Bravo G, Kanagal‐Shamanna R, Benton CB, et al. Genomic context and TP53 allele frequency define clinical outcomes in TP53‐mutated myelodysplastic syndromes. Blood Adv. 2020;4(3):482‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang G, Capo‐Chichi JM, Liu A, et al. Acute myeloid leukemia with myelodysplasia‐related changes diagnosed with multilineage dysplasia alone demonstrates a superior clinical outcome. Hum Pathol. 2020;104:117‐126. [DOI] [PubMed] [Google Scholar]
- 15. Gupta V, Kennedy JA, Capo‐Chichi JM, et al. Genetic factors rather than blast reduction determine outcomes of allogeneic HCT in BCR‐ABL‐negative MPN in blast phase. Blood Adv. 2020;4(21):5562‐5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alduaij W, McNamara CJ, Schuh A, et al. Clinical utility of next‐generation sequencing in the Management of Myeloproliferative Neoplasms: a single‐center experience. HemaSphere. 2018;2(3):e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas M, Sukhai MA, Zhang T, et al. Integration of technical, bioinformatic, and variant assessment approaches in the validation of a targeted next‐generation sequencing panel for myeloid malignancies. Arch Pathol Lab Med. 2017;141(6):759‐775. [DOI] [PubMed] [Google Scholar]
- 18. Grob T, Al Hinai ASA, Sanders MA, et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high‐risk myelodysplastic syndrome. Blood. 2022;139(15):2347‐2354. [DOI] [PubMed] [Google Scholar]
- 19. Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125(9):1367‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of Haematolymphoid Tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703‐1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arber DA, Orazi A, Hasserjian RP, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lancet JE, Uy GL, Cortes JE, et al. CPX‐351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684‐2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lancet JE, Uy GL, Newell LF, et al. CPX‐351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high‐risk or secondary acute myeloid leukaemia: 5‐year results of a randomised, open‐label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481‐e491. [DOI] [PubMed] [Google Scholar]
- 24. Tzogani K, Penttilä K, Lapveteläinen T, et al. EMA review of daunorubicin and cytarabine encapsulated in liposomes (Vyxeos, CPX‐351) for the treatment of adults with newly diagnosed, therapy‐related acute myeloid leukemia or acute myeloid leukemia with myelodysplasia‐related changes. Oncologist. 2020;25(9):e1414‐e1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Przespolewski A, Goldberg AD, Talati C, et al. Safety and efficacy of CPX‐351 in younger patients < 60 years old with secondary acute myeloid leukemia: an updated analysis. Blood. 2021;138(Supplement 1):1264 Available from: https://ashpublications.org/blood/article/138/Supplement1/1264/481179/Safety‐and‐Efficacy‐of‐CPX‐351‐in‐Younger‐Patients [DOI] [PubMed] [Google Scholar]
- 26. Goldberg AD, Talati C, Desai P, et al. TP53 mutations predict poorer responses to CPX‐351 in acute myeloid leukemia. Blood. 2018;132(Supplement 1):1433 Available from: https://ashpublications.org/blood/article/132/Supplement1/1433/262925/TP53‐Mutations‐Predict‐Poorer‐Responses‐to‐CPX‐351 [Google Scholar]
- 27. Chiche E, Rahme R, Bertoli S, et al. Real‐life experience with CPX‐351 and impact on the outcome of high‐risk AML patients: a multicentric French cohort. Blood Adv. 2021;5(1):176‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kadia TM, Jain P, Ravandi F, et al. TP53 mutations in newly diagnosed acute myeloid leukemia: Clinicomolecular characteristics, response to therapy, and outcomes. Cancer. 2016;122(22):3484‐3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grossmann V, Schnittger S, Kohlmann A, et al. A novel hierarchical prognostic model of AML solely based on molecular mutations. Blood. 2012;120(15):2963‐2972. Available from:. https://ashpublications.org/blood/article/120/15/2963/30596/A‐novel‐hierarchical‐prognostic‐model‐of‐AML [DOI] [PubMed] [Google Scholar]
- 30. Lindsley RC, Gibson CJ, Murdock HM, et al. Genetic characteristics and outcomes by mutation status in a phase 3 study of CPX‐351 versus 7+3 in older adults with newly diagnosed, high‐risk/secondary acute myeloid leukemia (AML). Blood. 2019;134(Supplement_1):15. Available from:. https://ashpublications.org/blood/article/134/Supplement_1/15/427820/Genetic‐Characteristics‐and‐Outcomes‐By‐Mutation [Google Scholar]
- 31. Ciurea SO, Chilkulwar A, Saliba RM, et al. Prognostic factors influencing survival after allogeneic transplantation for AML/MDS patients with TP53 mutations. Blood. 2018;131(26):2989‐2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poiré X, Labopin M, Maertens J, et al. Allogeneic stem cell transplantation in adult patients with acute myeloid leukaemia and 17p abnormalities in first complete remission: a study from the acute leukemia working party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). J Hematol Oncol. 2017;10(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lehmann S, Bykov VJN, Ali D, et al. Targeting p53 in vivo: a first‐in‐human study with p53‐targeting compound APR‐246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30(29):3633‐3639. [DOI] [PubMed] [Google Scholar]
- 34. Sallman DA, DeZern AE, Garcia‐Manero G, et al. Eprenetapopt (APR‐246) and Azacitidine in TP53‐mutant myelodysplastic syndromes. J Clin Oncol. 2021;39(14):1584‐1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mishra A, Tamari R, DeZern AE, et al. Phase II trial of Eprenetapopt (APR‐246) in combination with Azacitidine (AZA) As maintenance therapy for TP53 mutated AML or MDS following allogeneic stem cell transplantation (SCT). Blood. 2021;138(Supplement 1):409 Available from: https://ashpublications.org/blood/article/138/Supplement1/409/478208/Phase‐II‐Trial‐of‐Eprenetapopt‐APR‐246‐in [Google Scholar]
- 36. Cluzeau T, Sebert M, Rahmé R, et al. Eprenetapopt plus Azacitidine in TP53‐mutated myelodysplastic syndromes and acute myeloid leukemia: a phase II study by the Groupe francophone des Myélodysplasies (GFM). J Clin Oncol. 2021;39(14):1575‐1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eladl E, Tremblay‐Lemay R, Rastgoo N, et al. Role of CD47 in hematological malignancies. J Hematol Oncol. 2020;13(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sallman DA, Asch AS, Al Malki MM, et al. The first‐in‐Class anti‐CD47 antibody Magrolimab (5F9) in combination with Azacitidine is effective in MDS and AML patients: ongoing phase 1b results. Blood. 2019;134(Supplement_1):569 Available from: https://ashpublications.org/blood/article/134/Supplement_1/569/426374/The‐First‐in‐Class‐Anti‐CD47‐Antibody‐Magrolimab [Google Scholar]
- 39. Sallman D, Asch A, Kambhampati S, et al. AML‐196: the first‐in‐Class anti‐CD47 antibody Magrolimab in combination with Azacitidine is well tolerated and effective in AML patients: phase 1b results. Clin Lymphoma Myeloma Leuk. 2021;21:S290. [Google Scholar]
- 40. Bernard E, Nannya Y, Hasserjian RP, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020;26(10):1549‐1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weinberg OK, Siddon AJ, Madanat Y, et al. TP53 mutation defines a unique subgroup within complex karyotype de novo and therapy‐related MDS/AML. Blood Adv. 2022;6(9):2847‐2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. WCT: Haematolymphoid. [cited 2022 Apr 7]. Available from: https://whobluebooks.iarc.who.int/structures/haematolymphoid/
- 43. Short NJ, Montalban‐Bravo G, Hwang H, et al. Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Adv. 2020;4(22):5681‐5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rücker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119(9):2114‐2121. [DOI] [PubMed] [Google Scholar]
- 45. DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quek L, Ferguson P, Metzner M, et al. Mutational analysis of disease relapse in patients allografted for acute myeloid leukemia. Blood Adv. 2016;1(3):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Döhner H, Dolnik A, Tang L, et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with azacitidine or conventional care. Leukemia. 2018;32(12):2546‐2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim K, Maiti A, Loghavi S, et al. Outcomes of TP53‐mutant acute myeloid leukemia with decitabine and venetoclax. Cancer. 2021;127(20):3772‐3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prochazka KT, Pregartner G, Rücker FG, et al. Clinical implications of subclonal TP53 mutations in acute myeloid leukemia. Haematologica. 2019;104(3):516‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang RQ, Chen CJ, Jing Y, et al. Characteristics and prognostic significance of genetic mutations in acute myeloid leukemia based on a targeted next‐generation sequencing technique. Cancer Med. 2020;9(22):8457‐8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haase D, Stevenson KE, Neuberg D, et al. TP53 mutation status divides myelodysplastic syndromes with complex karyotypes into distinct prognostic subgroups. Leukemia. 2019;33(7):1747‐1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morita K, Kantarjian HM, Wang F, et al. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J Clin Oncol. 2018;36(18):1788‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jongen‐Lavrencic M, Grob T, Hanekamp D, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018;378(13):1189‐1199. [DOI] [PubMed] [Google Scholar]
- 54. Bacher U, Haferlach T, Schoch C, Kern W, Schnittger S. Implications of NRAS mutations in AML: a study of 2502 patients. Blood. 2006;107(10):3847‐3853. [DOI] [PubMed] [Google Scholar]
- 55. Bowen DT, Frew ME, Hills R, et al. RAS mutation in acute myeloid leukemia is associated with distinct cytogenetic subgroups but does not influence outcome in patients younger than 60 years. Blood. 2005;106(6):2113‐2119. [DOI] [PubMed] [Google Scholar]
- 56. Ball BJ, Hsu M, Devlin SM, et al. The prognosis and durable clearance of RAS mutations in patients with acute myeloid leukemia receiving induction chemotherapy. Am J Hematol. 2021;96(5):E171‐E175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kurzer JH, Weinberg OK. PHF6 mutations in hematologic malignancies. Front Oncol. 2021;11:704471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mrózek K, Eisfeld AK, Kohlschmidt J, et al. Complex karyotype in de novo acute myeloid leukemia: typical and atypical subtypes differ molecularly and clinically. Leukemia. 2019;33(7):1620‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
Data are available on request from the corresponding author.


