FIGURE 4.
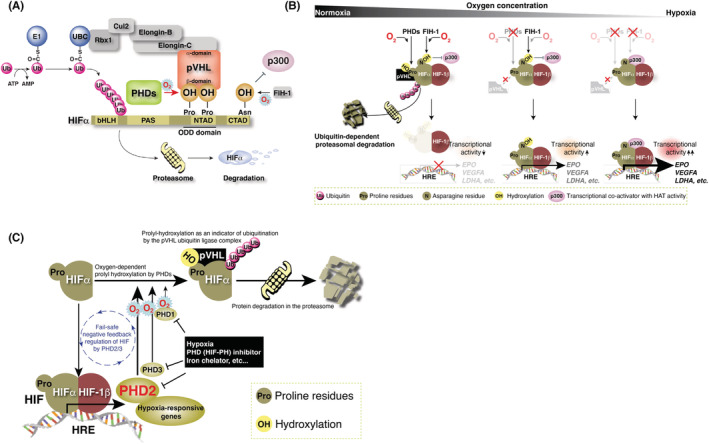
Regulation of hypoxia inducible factor (HIF) protein abundance by VHL gene product pVHL. (A) pVHL forms an E3 ubiquitin ligase complex with Elongin‐B/C, Cul2, and Rbx1. The β‐domain of pVHL recognizes one or two hydroxylated proline residue(s) in the N‐terminal transcriptional activation domain (NTAD) of HIFα, binds to HIFα, and targets HIFα for ubiquitin‐dependent protein degradation at the proteasome. (B) Molecular mechanism of oxygen sensing and adaptation. Prolyl hydroxylases (PHDs) negatively regulate the protein abundance of HIFα via the prolyl‐hydroxylation‐mediated ubiquitin‐proteasome pathway, and factor inhibiting HIF‐1 (FIH‐1) negatively regulates the transcriptional activity of HIF through asparaginyl hydroxylation. Both regulate the hypoxic response. (C) Fail‐safe negative feedback regulation of HIF by PHD2‐3. PHD1 hydroxylates HIFα independent of HIF activation. All three PHDs cooperatively hydroxylate HIFα to suppress constitutive activation of HIF.
