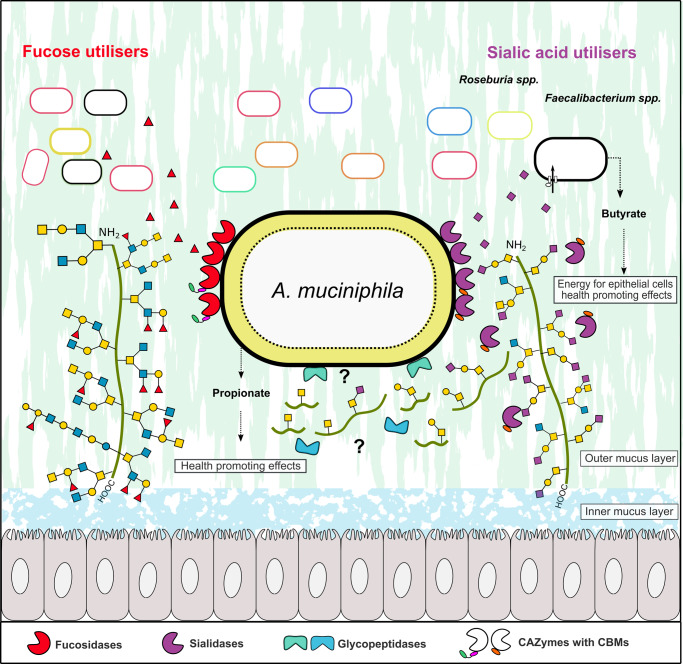
Fig. 5. Model for mucin defucosylation and desialylation by A. muciniphila and sharing of fucose and sialic acid with mucus adherent butyrogenic gut microbiota.
The Grey cells represent epithelial colonocytes, and the green line represents the mucin peptide backbone. The fucosidase activity was detected mainly in the intact A. muciniphila cell fraction, which is consistent with an extracellular cell-attached localization. Sialidase activity on 3´-sialyllactose and 6´-sialyllactose was detected both in intact cells and culture supernatant, which is consistent with the cell attachment of at least one sialidase and secretion of at least one sialidase. The removal of sialic acid exposes the recognition motifs for glycopeptidases that have been shown to cleave primarily adjacent to non-sialylated T and/or Tn epitopes, thereby enabling the cleavage of the mucin peptide backbone. The localization of the glycopeptidases has not been experimentally proven, but it is inferred, as intact mucin is too large to be internalized. The released sialic acid was not utilised by A. muciniphila and was shown to confer butyrate production by sialic-acid utilising model butyrogenic Clostridia. Similarly, fucose did confer meaningful growth of A. muciniphila (see Fig. 4d), making it available for cross-feeding to fucose utilisers. The figure background is inspired from figure 6 in ref. 47, cited in the present study.