Figure EV1. Rpn4‐dependent proteasome induction.
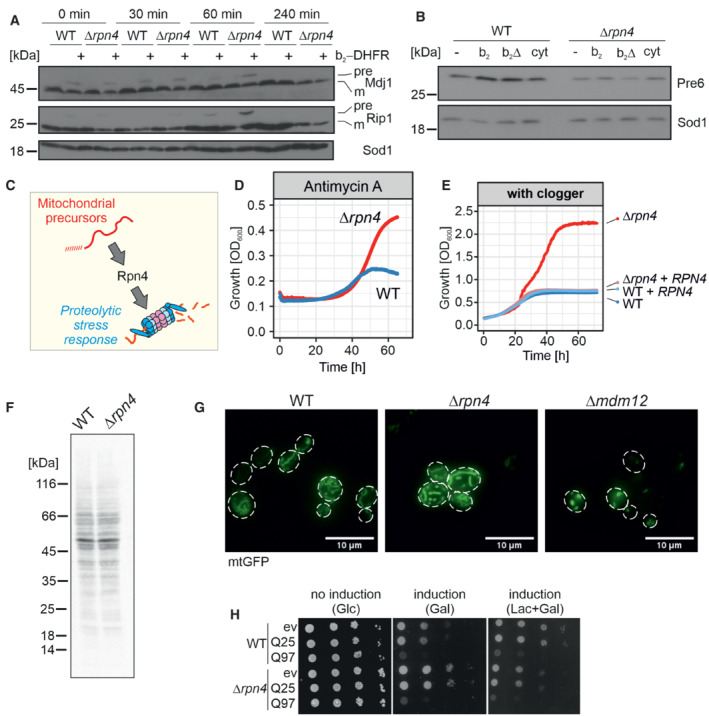
- After 4.5 h of clogger expression in wild‐type and Δrpn4 cells, the medium was exchanged for a noninducing lactate medium. Precursor (pre) and mature (m) forms of the mitochondrial proteins Mdj1 and Rip1 were visualized by Western blotting. Sod1 was used as a loading control.
- After 4.5 h expression of clogger and control, protein levels of the proteasome subunit Pre6 were visualized by Western Blotting. Please note, that in Δrpn4 cells, clogger expression does not lead to Pre6 induction. Sod1 served as a loading control.
- Schematic representation of Rpn4‐dependent proteasome induction after mitoprotein‐induced stress.
- Cells of the indicated strains were grown to log phase and diluted in lactate medium to 0.1 OD600. After the addition of 100 μg/ml antimycin A, growth was monitored upon constant agitation. Since antimycin A loses its activity over time, cells start growing once the membrane potential reaches a level that allows mitochondrial biogenesis. Δrpn4 cells escaped the antimycin A‐mediated growth inhibition more efficiently than wild‐type cells.
- Clogger‐expressing wild‐type and Δrpn4 cells harboring empty or Rpn4‐expression plasmids were grown over time in lactate plus 0.5% galactose medium.
- Wild‐type and Δrpn4 cells were grown in lactate medium to log phase. 2 μl of 22 μCi of 35S‐methionine was added to the cell suspension. After 5 min, cells were harvested, lysed, and subjected to SDS gel electrophoresis and autoradiography.
- The indicated strains were transformed with plasmids expressing mitochondria‐targeted GFP (mtGFP). The mitochondrial network was visualized by microscopy upon growth on galactose. Cells lacking Mdm12 were used as a control for a strain showing defective mitochondrial network formation (Dimmer et al, 2002). Scale bars, 10 μm.
- Yeast cells expressing a fusion protein of the N‐terminal region of human huntingtin with different lengths of glutamine residues (Q25‐GFP and Q97‐GFP, respectively; Schlagowski et al, 2021) and GFP under control of the galactose‐inducible promoter, or a control for comparison were grown to mid‐log phase on noninducing glucose medium. Ten‐fold serial dilutions were dropped on glucose (no induction), galactose (induction), or lactate with 0.5% galactose medium (induction).
Source data are available online for this figure.
