Abstract
Renal fibrosis is the final manifestation of chronic kidney disease (CKD) regardless of etiology. Hypoxia-inducible factor-2 alpha (HIF-2α) is an important regulator of chronic hypoxia, and the late-stage renal tubular HIF-2α activation exerts protective effects against renal fibrosis. However, its specific role in progressive renal fibrosis remains unclear. Here, we investigated the effects of the long-term tubular activation of HIF-2α on renal function and fibrosis, using in vivo and in vitro models of renal fibrosis. Progressive renal fibrosis was induced in renal tubular epithelial cells (TECs) of tetracycline-controlled HIF-2α transgenic (Tg) mice and wild-type (WT) controls through a 6-week adenine diet. Tg mice were maintained on doxycycline (DOX) for the diet period to induce Tg HIF-2α expression. Primary TECs isolated from Tg mice were treated with DOX (5 μg/ml), transforming growth factor-β1 (TGF-β1) (10 ng/ml), and a combination of both for 24, 48, and 72 hr. Blood was collected to analyze creatinine (Cr) and blood urea nitrogen (BUN) levels. Pathological changes in the kidney tissues were observed using hematoxylin and eosin, Masson’s trichrome, and Sirius Red staining. Meanwhile, the expression of fibronectin, E-cadherin and α-smooth muscle actin (α-SMA) and the phosphorylation of p38 mitogen-activated protein kinase (MAPK) was observed using western blotting. Our data showed that serum Cr and BUN levels were significantly lower in Tg mice than in WT mice following the adenine diet. Moreover, the protein levels of fibronectin and E-cadherin and the phosphorylation of p38 MAPK were markedly reduced in the kidneys of adenine-fed Tg mice. These results were accompanied by attenuated fibrosis in Tg mice following adenine administration. Consistent with these findings, HIF-2α overexpression significantly decreased the expression of fibronectin in TECs, whereas an increase in α-SMA protein levels was observed after TGF-β1 stimulation for 72 hr. Taken together, these results indicate that long-term HIF-2α activation in CKD may inhibit the progression of renal fibrosis and improve renal function, suggesting that long-term renal HIF-2α activation may be used as a novel therapeutic strategy for the treatment of CKD.
Keywords: Chronic kidney disease, Fibrosis, Hypoxia-inducible factor-2 alpha, Mitogen-activated protein kinase, Renal tubular epithelial cells
INTRODUCTION
An imbalance between oxygen supply and consumption leads to renal tissue hypoxia, which is the central player in renal fibrosis and the final common pathway of chronic kidney disease (CKD) (1, 2). Renal tubular epithelial cells (TECs) are particularly prone to hypoxic injury owing to their high metabolic activity and oxygen demand (3). Persistent hypoxic injury of TECs induces pro-fibrogenic events, including renal inflammation, fibroblast activation, and cell cycle arrest, resulting in tissue destruction (4).
Hypoxia-inducible factor (HIF) is a crucial intermediate in the regulation of adaptive responses to hypoxia in various pathophysiological processes of kidney diseases (5). HIFs are heterodimers consisting of an oxygen-labile α-subunit (HIF-1, HIF-2, and HIF-3) and a common stable β-subunit (6). The α-subunit of HIF escapes prolyl hydroxylase-mediated hydroxylation under hypoxia, translocates to the nucleus, and combines with the β-subunit (7). HIF-α/β heterodimer binds to hypoxia response elements and regulates the transcription of HIF target genes (8). HIF is activated by these mechanisms, resulting in the expression of various protective factors, including vascular endothelial growth factor (VEGF), erythropoietin (EPO) and glucose transporter-1 (7).
Stable overexpression of HIF-1α in renal epithelial cells inhibits tubulointerstitial fibrosis in the remnant kidney and unilateral ureteral obstruction models (9, 10). However, contradictory results indicate that HIF activation in the early recovery phase of CKD exerts no beneficial effects (11). Moreover, HIF activation may be beneficial or detrimental to the general outcome in patients from a renal perspective (12). There is still controversy about the effects of HIF activation on CKD and acute kidney injury (AKI) (13, 14). Therefore, previously, we investigated the effects of HIF-2α activation on renal fibrosis based on its activation timing in inducible tubule-specific transgenic (Tg) mice with nondiabetic CKD. Based on these results, we hypothesized that the selective tubular activation of HIF-2α may have dual effects on renal fibrosis in CKD (11).
Although many recent studies have focused only on the effects of acute hypoxia, others have explored the effects of long-term hypoxia. Thus, we aimed to investigate the long-term effect of HIF-2α activation on CKD based on the timing of HIF-2α activation, as determined in our previous study.
RESULTS
Long-term tubular overexpression of HIF-2α improved renal dysfunction in a CKD model
We used our previously established (Tg) mouse line (PAX8-rtTA, tetO-Cre and HIF2dPA-HA) that overexpressed HIF-2α in renal tubular cells after the administration of doxycycline (DOX) (11).
To test the effects of renal tubular HIF-2α overexpression on progressive renal fibrosis in CKD mice, we induced an experimental CKD model based on a 6-week diet containing 0.2% adenine in DOX-administered wild-type (WT) and Tg mice (15).
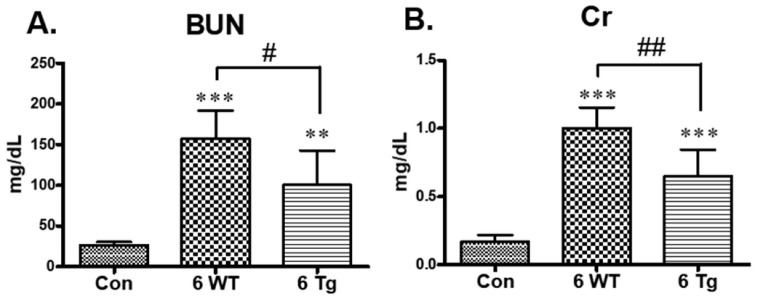
Blood urea nitrogen (BUN) levels were significantly higher in WT CKD mice fed an adenine diet than in control mice fed a standard diet (control). Similarly, serum creatinine (Cr) levels were significantly higher in WT CKD mice than in the controls (Fig. 1). However, these effects were partially abolished in the Tg CKD mice (Fig. 1).
Fig. 1.
Effects of long-term hypoxia-inducible factor-2 alpha (HIF-2α) activation on renal function in the chronic kidney disease (CKD) model. (A) Serum blood urea nitrogen (BUN) and (B) creatinine (Cr) levels were determined in the standard diet-fed control mice, and the 6-week doxycycline (DOX) + 0.2% adenine diet-fed wild-type (WT) and transgenic (Tg) CKD mice. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the controls; #P < 0.05 between the two groups.
Long-term tubular overexpression of HIF-2α inhibited renal fibrosis during CKD progression
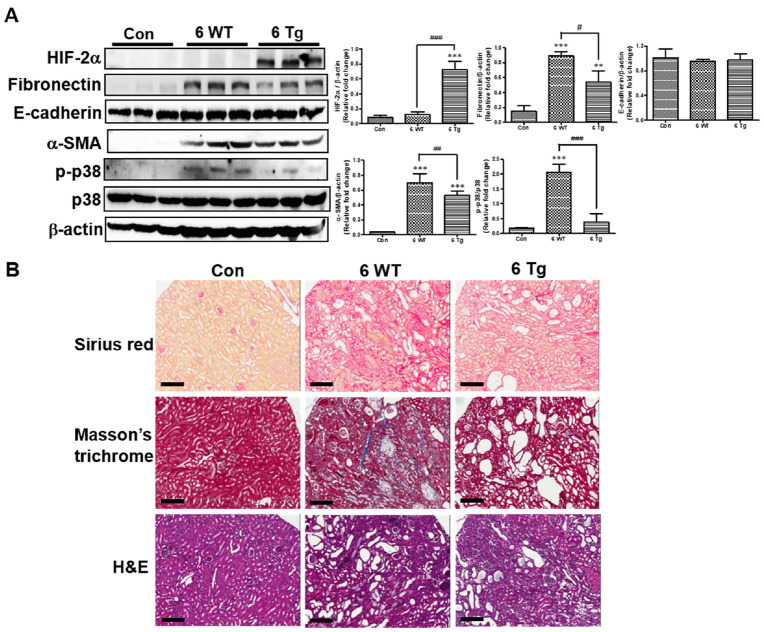
To assess the effects of renal tubular HIF-2α overexpression on progressive renal fibrosis in CKD, we first demonstrated that the renal expression of HIF-2α was induced in response to DOX administration in Tg CKD mice, but not in WT CKD mice (Fig. 2A). Next, we analyzed the markers associated with fibrosis in the kidneys of DOX-exposed mice. Fibronectin and α-smooth muscle actin (α-SMA) protein expression levels were significantly higher in WT CKD mice than in control mice and significantly lower in Tg CKD mice than in WT CKD mice. In addition, no significant difference was detected in the protein levels of epithelial cell marker E-cadherin between WT and Tg CKD mice.
Fig. 2.
Effects of long-term HIF-2α activation on renal fibrosis in the CKD model. (A) HIF-2α, fibronectin, E-cadherin, α-smooth muscle actin (α-SMA), and phosphorylated-p38 (p-p38) protein levels in the kidneys of standard diet-fed control mice and 6-week DOX + 0.2% adenine diet-fed WT and Tg mice were determined using western blotting (left panel). Band intensities in the western blot were quantitated by computerized densitometry blotting (right panel). *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the controls; #P < 0.05 between the two groups. (B) Representative kidney sections from the same mice as in (A) stained with Sirius Red, Masson’s trichrome, and hematoxylin and eosin (H&E). Scale bar, 20 μm.
To further confirm the effects of HIF-2α on the mitogen-activated protein kinase (MAPK) signaling pathway, we performed immunoblot analysis and observed that the phosphorylated-p38 (p-p38) levels were significantly higher in WT CKD mice than in control mice and significantly lower in Tg CKD mice than in WT CKD mice.
Masson’s trichrome and Sirius Red staining revealed that renal fibrosis patterns were similar to those observed by western blotting for fibronectin protein expression (Fig. 2B). Disease severity was confirmed histologically in WT CKD mice, and the condition of Tg CKD mice was improved.
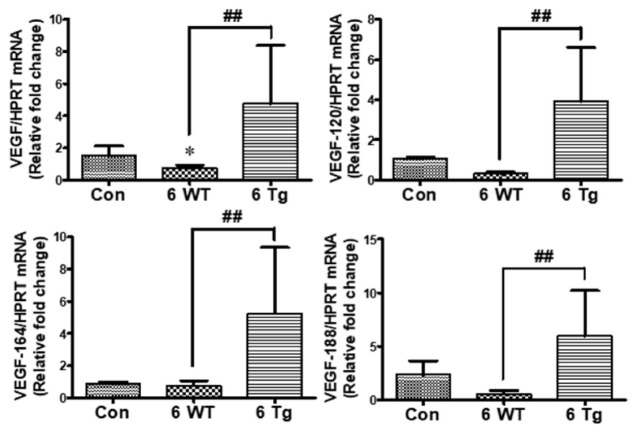
VEGF mRNA levels were significantly increased by long-term tubular overexpression of HIF-2α in Tg CKD mice
HIF-2α is the major regulator of VEGF and EPO induction, and both HIF-1 and HIF-2 are expressed in tissues, such as the brain, liver, and kidney (5, 16). Therefore, we analyzed the mRNA levels of total VEGF and its isoforms (120, 164, 188) using quantitative real-time polymerase chain reaction (qPCR) and found that VEGF mRNA levels were significantly higher in Tg CKD mice than in control mice. In addition, VEGF mRNA levels were slightly lower in WT CKD mice than in controls, but the difference was not statistically significant (Fig. 3).
Fig. 3.
Effect of changes in the mRNA levels of total vascular endothelial growth factor (VEGF) and its isoforms (VEGF-120, 164, 188) on tubular long-term HIF-2α overexpression in CKD mice. The mRNA levels of VEGF and its isoforms were determined via qPCR of RNA from the kidneys of standard diet-fed control mice, and 6-week DOX + 0.2% adenine diet-fed WT and Tg mice. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the controls; #P < 0.05, ##P < 0.01 and ###P < 0.001 between the two groups.
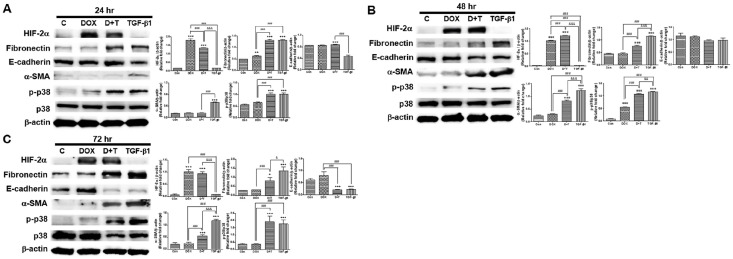
Long-term HIF-2α overexpression attenuated transforming growth factor-β1 (TGF-β1)-induced fibrosis and p38 activation in TECs
We have previously found that HIF-2α activation attenuates renal fibrosis (11), which is consistent with the in vivo results of this study. Therefore, we assessed the long-term effects of HIF-2α overexpression in vitro using TGF-β1-stimulated primary TECs from the Tg mice used for in vivo experiments. HIF-2α protein was overexpressed only in DOX-treated TECs (Fig. 4). TGF-β1 induced a strong increase in the protein levels of the fibrotic markers, fibronectin and α-SMA, in TECs. However, DOX treatment significantly suppressed fibronectin induction after 48 hr and was inhibited α-SMA induction after 24 hr. Additionally, p-p38 level was decreased at 48 hr following DOX and TGF-β1 treatment compared that after TGF-β1 treatment (Fig. 4). The E-cadherin protein levels were decreased by DOX in TGF-β1-treated cells at 24 hr, but it had no effect at 48 and 72 hr (Fig. 4). These results indicate that long-term HIF-2α overexpression by DOX in TECs markedly attenuated the fibrotic effects of TGF-β1 stimulation, consistent with the in vivo findings.
Fig. 4.
Effect of HIF-2α overexpression by DOX induction in renal tubular epithelial cells (TECs) under stimulation with transforming growth factor-β1 (TGF-β1) for 24, 48, and 72 hr. (A-C) HIF-2α, fibronectin, E-cadherin, α-SMA, and p-p38 protein levels were determined using western blotting of TECs treated with 10 ng/ml of TGF-β1 and 5 μg/ml of DOX for the indicated period (left panel). Band intensities were quantified via computerized densitometry (right panel). *P < 0.05 and ***P < 0.001 compared to the controls; #P < 0.05 and ##P < 0.01 between the two groups.
DISCUSSION
These results indicate that long-term tubular HIF-2α overexpression inhibits renal fibrosis in CKD and that HIF-2α overexpression by DOX attenuated the degree of TGF-β1 stimulation in primary TECs.
The roles of HIF activation in CKD and AKI have been extensively studied (5, 6); however, clear conclusions are missing, and most opinions differ. Selective tubular activation of HIF-2α has dual effects on renal fibrosis (11).
HIF activation regulates various biological processes and renal functions, and its effects on CKD are well known (4). HIF activation in a diabetes model was suppressed by increased oxidative stress (17), and prolyl hydroxylase (PHD) inhibitors ameliorated CKD progression in a remnant kidney model (16). Furthermore, von Hippel-Lindau tumor suppressor deletion promoted the development of interstitial fibrosis in a 5/6 nephrectomy model (16). Further, activation of HIF-1α in the renal epithelial cells of mice attenuated the progression of tubulointerstitial fibrosis (10). HIF activation via pretreatment with a PHD inhibitor also ameliorated acute renal failure induced by ischemia/reperfusion in rats (18).
We studied the effect of renal fibrosis on HIF-2α long-term activation in renal tubules by creating a 0.2% adenine-induced CKD model mimicking many aspects of human CKD (19). The levels of fibronectin, a marker of fibrosis, were increased in the 0.2% adenine-induced CKD mouse model compared with that in the control, whereas they were significantly reduced in Tg CKD mice compared with that in WT CKD mice. Additionally, Cr and BUN levels decreased in Tg CKD mice as a result of renal function.
The Tg mice used in this study carried three transgenes, tetO-Cre (cre-recombinase driven by tetracycline-responsive promoter element), PAX8-rtTA (reverse tetracycline-dependent transactivator driven by PAX8 promoter), and hemagglutinin (HA)-tagged HIF2dPA (HA-tagged HIF2α variant that escapes recognition by the von Hippel-Lindau tumor suppressor gene product [pVHL]) (20). The PAX8 promoter in Tg mice was used to restrict DOX-mediated HIF-2α activation within renal tubular cells, one of the main sources of VEGF expression in the kidneys. Changes in both HIF-1α and HIF-2α levels correlate with tissue hypoxia, which is associated with a protein known to upregulate VEGF. In addition, the HIF protein can explain the integrity of peritubular capillaries in relation to VEGF expression (21).
We have previously shown that the mRNA levels of VEGF and its isoforms (120, 164, 188) were significantly higher in HIF-2α Tg mice 4 weeks after CKD induction (9). In the current study, the levels of these mRNAs were significantly increased in Tg CKD mice and were higher in the 6-week Tg CKD mice than in the 4-week Tg CKD mice. Therefore, we suggest that sustained activation of HIF-2α during CKD development may lead to the release of VEGF while inhibiting renal fibrosis and CKD progression.
We previously reported that HIF-2α protein expression levels were increased in a time-dependent manner by DOX treatment of TECs isolated from the kidney cortex of Tg mice (11). Therefore, we used a DOX-treated primary TECs overexpressing HIF-2α to examine the intracellular regulatory mechanism of HIF-2α in renal fibrosis induced by TGF-β1 stimulation, which could be used as a valuable model for in vitro studies. In the present study, we confirmed that HIF-2α protein expression levels were increased in the DOX-alone treated group, whereas the fibronectin levels were decreased upon treatment with DOX and TGF-β1, which act as mediators of renal fibrosis. To determine whether epithelial mesenchymal transition (EMT) occurred during the experiment, we examined E-cadherin and α-SMA protein levels using western blotting. EMT occurred at 24 hr and then decreased over time at 48 and 72 hr. The relationship between renal fibrosis and the MAPK pathway is well known; however, the precise roles of MAPKs in the renal cell response to hypoxia are unknown (22). Next, we evaluated the p-p38 levels in TECs treated with DOX and TGF-β1. We found that EMT first occurred at 24 hr and gradually disappeared over time, whereas p-p38 levels increased over time. Although underlying mechanism remains unknown, our results indicate that long-term HIF-2α activation could improve renal fibrosis.
In conclusion, long-term tubular overexpression of HIF-2α improved renal function and fibrosis in Tg CKD model. In addition, the levels of VEGF and its isoforms were significantly higher in Tg CKD mice than in control and WT CKD mice, suggesting that sustained activation of HIF-2α during CKD development leads to the release of VEGF and delays renal fibrosis. This in vivo study was followed by an in vitro study in which TECs were isolated from Tg mice and stimulated with TGF-β1 to confirm similar results.
Taken together, these findings show that long-term HIF-2α activation in CKD may inhibit the progression of renal fibrosis and improve renal function, suggesting that long-term renal HIF-2α activation may be a potential therapeutic strategy for CKD.
MATERIALS AND METHODS
Animal models
PAX8-rtTA, tetO-Cre, and HIF2dPA-HA Tg mice were obtained from the Samuel Lunenfeld Research Institute at Mount Sinai Hospital, Toronto, ON, Canada. The animal care guidelines and all methods used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the School of Medicine, Ewha Woman’s University (ESM 12-0201, ESM14-0265).
Six- to eight-week male mice were used for all animal experiments and were maintained under temperature- and light-controlled conditions (20-23°C, 12 hr light/dark cycle) and received food and water ad libitum. To obtain renal tubular cell-specific HIF-2α-overexpressing mice, DOX (Sigma Chemical Co., St. Louis, MO, USA) was added to drinking water at a concentration of 2 mg/ml for at least three days. To induce renal fibrosis and CKD, the mice were fed a custom ready-made diet (Central Lab Animal Inc., Korea) containing 0.2% (w/w) adenine (A2786; Sigma Chemical Co., St. Louis, MO, USA) for 6 weeks. Tg and WT mice were divided into three experimental groups: (i) WT mice fed a standard diet (control), (ii) WT mice fed a 0.2% (w/w) adenine-containing diet with DOX for 6 weeks, and (iii) Tg mice fed a 0.2% (w/w) adenine-containing diet with DOX for 6 weeks. Body weight was measured weekly.
All mice were anesthetized via intraperitoneal injection of a mixture of Zoletil (30 mg/kg; Virbac Laboratories, Carros, France) and Rompun (20 mg/kg; Bayer Korea, Ansan, Korea) and sacrificed at 6 weeks. Blood and kidney samples were collected for further analyses. The generation and genotyping of Tg mice for PAX8-rtTA, tetO-Cre, and HIF2dPA-HA have been described previously (11).
Serum chemistry analysis
Blood was collected via cardiac puncture at the time of sacrifice and placed in Vacutainer SST tubes (BD-belliver Industrial Estate, UK) without an anticoagulant (to obtain serum) or with EDTA for estimating renal function. Aliquots of serum were immediately stored at −80°C until further tests were performed. Serum Cr and BUN levels were determined using an automated analyzer (Cobase c502; Roche, Germany) for routine chemistry at the Seoul Medical Science Institute.
Histology
Kidney samples were intracardially perfused with normal saline (0.9% NaCl) and then with 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline PBS (pH 7.4). After perfusion, the kidneys were removed, placed in 4% PFA overnight at 4°C, and embedded in paraffin. Paraffin-embedded kidneys were sectioned at a thickness of 4 μm and stained with Masson’s trichrome (25088; Polysciences, Inc.,Warrington, PA, USA) or Sirius Red (ab150681; Abcam, Cambridge, UK) for the qualitative evaluation of renal fibrosis. Hematoxylin and eosin (H&E) (Sigma-Aldrich, St Louis, MO, USA) staining was performed to detect technical artifacts and histopathological changes. All staining procedures were performed according to the manufacturers’ instructions and photomicrographs were acquired using Aperio ImageScope (Aperio Technologies, Inc., Vista, CA, USA).
Isolation and culture of TECs
TECs were isolated from male Tg mice (PAX8-rtTA; tetO-Cre; HA-HIF2dPA; aged ≤ 4 weeks) using a previously described method. Briefly, the mice were anesthetized, and the kidneys were immediately removed, placed in 1 ml of ice-cold Dulbecco’s PBS (DPBS), and minced into pieces of less than 1 mm3. Fragments were transferred to collagenase solution I (1 mg/ml in DPBS; 9001-12-1; Gibco-Invitrogen, Carlsbad, CA, USA) and digested in a shaking incubator at 37°C for 30 min. The supernatant was resuspended by pipetting into the Roswell Park Memorial Institute (RPMI) 1640 medium, passed through a 100-μm nylon mesh, and centrifuged at 3,000 × g for 10 min. The pellet was placed in sterile red blood cell lysis buffer (8.26 g NH4Cl, 1 g KHCO3, and 0.037 g/L EDTA/ddH2O) and kept on ice for 3 min. The pellet was centrifuged and washed twice with the culture medium (RPMI 1640 medium consisting of 10% FBS, 1% penicillin-streptomycin, and 20 ng/ml of epithelial growth factor [EGF; Sigma Chemical Co., St. Louis, MO, USA]) (6). Primary TECs isolated from live mice were seeded in plates and incubated at 37°C in a 5% CO2 incubator. The medium was changed every two days until the cells reached approximately 80% confluence.
The cultured cells were then divided into the following four groups: (i) Con, (ii) Dox (5 μg/ml) for the induction of HIF-2α, (iii) Dox (5 μg/ml) + TGF-β1 (10 ng/ml), and (iv) TGF-β1 (10 ng/ml). After 24, 48, and 72 hr, the cells were harvested for subsequent experiments.
Western blotting
Homogenized whole kidney or cultured cell pellets were lysed in radioimmunoprecipitation assay lysis buffer (GeneDEPOT, USA) and quantified using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). Equal amounts of protein were separated by SDS-PAGE and then transferred to a PVDF membranes (BioRad Laboratories Inc., Hercules, CA, USA). The membrane were incubated overnight at 4°C with the primary antibodies as followed: anti-HIF-2α (rabbit, 1:500; Novus), anti-fibronectin (rabbit, 1:1000; Dako), anti-E-cadherin (mouse, 1:1000; BD Biosciences), anti-α-SMA (rabbit, 1:1000; Abcam), anti-p-p38 (rabbit, 1:500; Cell Signaling), anti-p38 (rabbit, 1:1000; Cell Signaling) and anti-β-actin (mouse, 1:1000; Sigma Chemical Co.) were used. Protein bands were detected using an enhanced chemiluminescence reagent (Thermo Scientific Pierce, Rockford, IL, USA) and LAS-4000 Imager (Fuji Film Corp., Tokyo, Japan). The band densities in the immunoblots were measured using ImageJ v.1.49 (NIH, Bethesda, MD, USA).
qPCR analysis
Total RNA was isolated from homogenized whole kidneys and cultured cell pellets using Easy-BLUE isolation reagent (iNtRON Biotechnology, Daejeon, Korea) according to the manufacturer’s protocol. cDNA was synthesized from 2 μg of purified total RNA using M-MLV reverse transcriptase (Enzyonomics, Seoul, Korea). qPCR analysis was performed using the ABI StepOne Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) and the QuantiFast SYBR Green PCR kit (Qiagen Inc., Hilden, Germany), according to general protocols. For quantitative analysis, all samples were normalized to hypoxanthine guanine phosphoribosyl transferase (HPRT) gene expression using the 2−DDCT value method. The primer sequences were as follows: VEGF forward, 5’-GTAC CTCCACCATGCCAAGT-3’; VEGF reverse, 5’-ACACAGGACGG CTTGAAGAT-3’; VEGF120 forward, 5’-AACGATGAAGCCCTGG AGTG-3’; VEGF120 reverse, 5’-TGAGAGGTCTGGTTCCCGA-3’; VEGF164 forward, 5’-AACGATGAAGCCCTGGAGTG-3’; VEGF164 reverse, 5’-GACAAACAAATGCTTTCTCCG-3’; VEGF188 forward, 5’-AACGATGAAGCCCTGGAGTG-3’; VEGF188 reverse, 5’-AA CAAGGCTCACAGTGAACG-3’; HPRT forward, 5’-CAGACTGAA GAGCTACTGTAATG-3’; HPRT reverse, 5’-CCAGTGTCAATTA TATCTTCAAC-3’.
Statistical analysis
GraphPad PRISM 6.0 (GraphPad, San Diego, CA, USA) was used for statistical analysis. All values are expressed as the mean ± standard error of the mean. Statistical analysis was performed using analysis of variance and the Kruskal-Wallis non-parametric test for multiple comparisons. Student’s t-test and the Mann-Whitney U-test were performed to compare the differences between the two groups. P-values < 0.05 were considered statistically significant.
Funding Statement
ACKNOWLEDGEMENTS This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2019R1I1A1A01054994 and 2019R1I1A1A01055061).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
References
- 1.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka S, Tanaka T, Nangaku M. Hypoxia and dysregulated angiogenesis in kidney disease. Kidney Dis (Basel) 2015;1:80–89. doi: 10.1159/000381515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu M, Ning X, Li R, et al. Signalling pathways involved in hypoxia-induced renal fibrosis. J Cell Mol Med. 2017;21:1248–1259. doi: 10.1111/jcmm.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Wei Q, Guo C, et al. Hypoxia, HIF, and associated signaling networks in chronic kidney disease. Int J Mol Sci. 2017;18:950. doi: 10.3390/ijms18050950.9c487a54c4e0480e8f23265a617b9d6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka T. Expanding roles of the hypoxia-response network in chronic kidney disease. Clin Exp Nephrol. 2016;20:835–844. doi: 10.1007/s10157-016-1241-4. [DOI] [PubMed] [Google Scholar]
- 7.Mimura I, Tanaka T, Nangaku M. Novel therapeutic strategy with hypoxia-inducible factors via reversible epigenetic regulation mechanisms in progressive tubulointerstitial fibrosis. Semin Nephrol. 2013;33:375–382. doi: 10.1016/j.semnephrol.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirakawa Y, Tanaka T, Nangaku M. Renal hypoxia in CKD; pathophysiology and detecting methods. Front Physiol. 2017;8:99. doi: 10.3389/fphys.2017.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Wang W, Zhang F, et al. Hypoxia inducible factor-1alpha mediates the profibrotic effect of albumin in renal tubular cells. Sci Rep. 2017;7:15878. doi: 10.1038/s41598-017-15972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong KH, Oh HJ, Lim BJ, et al. Selective tubular activation of hypoxia-inducible factor-2alpha has dual effects on renal fibrosis. Sci Rep. 2017;7:11351. doi: 10.1038/s41598-017-11829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshmukh AB, Patel JK, Prajapati AR, Shah S. Perspective in chronic kidney disease: targeting hypoxia-inducible factor (HIF) as potential therapeutic approach. Ren Fail. 2012;34:521–532. doi: 10.3109/0886022X.2011.653754. [DOI] [PubMed] [Google Scholar]
- 13.Nangaku M, Rosenberger C, Heyman SN, Eckardt KU. Regulation of hypoxia-inducible factor in kidney disease. Clin Exp Pharmacol Physiol. 2013;40:148–157. doi: 10.1111/1440-1681.12005. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol. 2014;307:F1187–F1195. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 15.Tani T, Orimo H, Shimizu A, Tsuruoka S. Development of a novel chronic kidney disease mouse model to evaluate the progression of hyperphosphatemia and associated mineral bone disease. Sci Rep. 2017;7:2233. doi: 10.1038/s41598-017-02351-6.33e6c805e8a7414997ed3c8e5081e7c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol Dial Transplant. 2011;26:1132–1137. doi: 10.1093/ndt/gfq832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JH, Jun JH, Shim JK, Shin EJ, Shin E, Kwak YL. Effects of post ischemia-reperfusion treatment with trimetazidine on renal injury in rats: insights on delayed renal fibrosis progression. Oxid Med Cell Longev. 2018;2018:1072805. doi: 10.1155/2018/1072805.10a8c327bf5a4413bb40bf024c9911dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nangaku M, Eckardt KU. Hypoxia and the HIF system in kidney disease. J Mol Med (Berl) 2007;85:1325–1330. doi: 10.1007/s00109-007-0278-y. [DOI] [PubMed] [Google Scholar]
- 19.Jia T, Olauson H, Lindberg K, et al. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol. 2013;14:116. doi: 10.1186/1471-2369-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim WY, Safran M, Buckley MR, et al. Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. EMBO J. 2006;25:4650–4662. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sulikowska B, Rutkowski B, Marszalek A, Manitius J. The role of interstitial changes in the progression of chronic kidney disease. Postepy Hig Med Dosw (Online) 2015;69:830–837. doi: 10.5604/17322693.1162570. [DOI] [PubMed] [Google Scholar]
- 22.Luo F, Shi J, Shi Q, He X, Xia Y. ERK and p38 upregulation versus Bcl-6 downregulation in rat kidney epithelial cells exposed to prolonged hypoxia. Cell Transplant. 2017;26:1441–1451. doi: 10.1177/0963689717720296.8c60fd46299749d18b7975c38d62d979 [DOI] [PMC free article] [PubMed] [Google Scholar]