Abstract
Mechanosensitive ion channels sense mechanical stimuli applied directly to the cellular membranes or indirectly through their tethered components, provoking cellular mechanoresponses. Among others, Piezo1 mechanosensitive ion channel is a relatively novel Ca2+-permeable channel that is primarily present in non-sensory tissues. Recent studies have demonstrated that Piezo1 plays an important role in Ca2+-dependent cell death, including apoptosis and ferroptosis, in the presence of mechanical stimuli. It has also been proven that cancer cells are sensitive to mechanical stresses due to higher expression levels of Piezo1 compared to normal cells. In this review, we discuss Piezo1-mediated cell death mechanisms and therapeutic strategies to inhibit or induce cell death by modulating the activity of Piezo1 with pharmacological drugs or mechanical perturbations induced by stretch and ultrasound.
Keywords: Apoptosis, Calcium ion (Ca2+), Ferroptosis, Mechanosensitive ion channel, Piezo1
INTRODUCTION
Mechanotransduction is a process whereby cells respond to mechanical forces (e.g., static pressure, membrane stretch, shear flow, substrate stiffness, tissue compression, and osmotic stress) and transduce them into biochemical signals, thereby influencing cell behavior and fate (1, 2). Among a variety of proteins involved in mechanotransduction, mechanosensitive (MS) ion channels, also known as mechanically gated ion channels, execute the initial mechanosensing step. MS ion channels change their conformations from a closed to an open form via directly sensing the mechanical stresses applied to the cellular membranes or through the associated nonmembrane components such as cytoskeleton (3-6). Diverse MS ion channel superfamilies, including the epithelial sodium channel/degenerin (ENaC/DEG), Piezo-type nonselective cation channels (e.g., Piezo1 and Piezo2), two pore-domain potassium ion channels (K2P/KCNK) (e.g., TREK/TRAAK), transient receptor potential (TRP) nonselective cation channels, and transmembrane protein 16/Anoctamin (TMEM16/Ano) Ca2+-activated chloride channels, are known (7). These channels possess different structures and mechanotransduction mechanisms with distinct biological roles. Notably, MS ion channels are not only expressed in sensory cells but also in non-sensory cells (8, 9), playing important roles in a broad range of cellular functions.
One of the most important roles of MS ion channels is to properly regulate cell death as dysregulated intracellular ion homeostasis results in cell death by triggering cell shrinkage (i.e., apoptosis) or swelling (i.e., necrosis) (10). Recent studies have demonstrated that the activation of MS ion channels mediates cell deaths led by mechanical stimulation (11-13). Uniaxial cyclic tensile strain induced apoptosis in mesenchymal stem cells by opening L-type voltage-gated Ca2+ channels that subsequently activated calpain protease and c-Jun N-terminal kinase (JNK), causing caspase-dependent DNA fragmentation (14). In addition, intracellular accumulation of Ca2+ was strain-dependent and correlated with apoptosis in aortic valve interstitial cells (15). The mechanical onset of apoptosis was prevented upon a treatment with gadolinium (Gd3+), which is an inhibitor of mechanically-gated cation channels (16, 17). As uncontrolled cell death can lead to multiple human diseases, including osteoarthritis, metabolic syndrome, and cancer (18, 19), targeting MS ion channels can have potential therapeutic effects. In this review, we focus on the mechanisms by which Piezo-type MS Ca2+-permeable channels regulate apoptosis and ferroptosis, the most typical and newest type of cell death, respectively. Furthermore, therapeutic strategies to inhibit or induce cell death by modulating the activity of Piezo channels using pharmacological drugs or mechanical stimuli are discussed.
MECHANOSENSITIVE ION CHANNELS
MS ion channels sense and transduce external and internal mechanical stimuli into different biochemical responses (20, 21). In response to the presence of mechanical forces in cell membranes, the MS ion channels open in a pore-like manner via a conformational change induced by membrane tension (i.e., stretch) or physical connections, the so-called spring-like tethers, with the extracellular matrix and cortical cytoskeleton. The bacterial large-conductance MS ion channel (MscL) is an example of one such channel, which opens by membrane tension to prevent cell lysis under osmotic stress (22). Eukaryotic MS ion channels were first documented in 1984 in the skeletal muscle of embryonic chicks using patch-clamp technique (23). About 37 years later, the 2021 Nobel Prize in Physiology or Medicine was awarded to David Julius and Ardem Patapoutian for their discovery of vertebrate thermo-sensory TRP channels and mechanosensory Piezo-type ion channels, respectively (24).
Piezo1 (a.k.a. Fam38A) and its paralog Piezo2 (a.k.a. Fam38B) were discovered in 2010 using a small interfering RNA (siRNA)-based screening and were named after the Greek “piesh” (pίesi) meaning pressure (25). There exists 47% identity in the amino acid sequence of the extracellular cap domain, which plays a decisive role in conferring the distinct kinetics of inactivation, between mouse Piezo1 and Piezo2 (25-27). The structure of Piezo channels resembles that of a three-bladed propeller and changes from a closed to an open state (i.e., pore-forming) upon mechanical stimulation, thereby allowing the influx of cations, particularly that of Ca2+, into the cytosol (27). Piezo channels are primarily located in the plasma membrane as well as in the endoplasmic reticulum (ER) and nuclear membranes, wherein these channels sense the membrane displacement that occur owing to regional and overall mechanical pressure (28-31). Since the discovery of Piezo channels that convert mechanical stimuli to intracellular signaling (i.e., mechanotransduction) via ion flux, which is critical for various biological processes, it has been cumulatively reported that Piezo channels play important regulatory roles in a number of cellular functions, including proliferation, migration, and differentiation (32-35).
THE ROLE OF PIEZO1 IN APOPTOSIS
Apoptosis is a mode of programmed cell death with distinct morphological features, including cell shrinkage, chromatin condensation leading to pyknosis (during the early phase), and plasma membrane blebbing followed by karyorrhexis and separation of cell fragments into apoptotic bodies (during the late phase) (36). The apoptotic bodies are subsequently phagocytosed and degraded by macrophages and parenchymal or neoplastic cells. Apoptosis normally acts as a homeostatic mechanism that maintains the cell population during development and aging or as a host defense mechanism against viral infections. Hence, inappropriate apoptosis is closely related with many human diseases, including cancers.
Loss of MS ion channel-mediated Ca2+ homeostasis can lead to apoptosis as cellular Ca2+ overload and perturbation of intracellular Ca2+ compartmentalization are well-known to be cytotoxic (37). Ca2+ released from stressed ER via ryanodine receptors (RyRs) and inositol-1,4,5-trisphosphate receptors (Ins(1,4,5)P3Rs) into the cytosol is taken up by mitochondria through a mitochondrial calcium uniporter (MCU). Once Ca2+ binds to cardiolipin at the inner mitochondrial membrane, cytochrome c (Cyt c) is dissociated from cardiolipin and is released through mitochondrial permeability transition pore (MPTP) opening into the cytosol, wherein Cyt c forms the apoptosome complex with apoptotic protease activating factor-1 (APAF-1) and procaspase-9; this promotes the activation of caspase-9 and subsequently caspase-3, leading to apoptosis (38-40). Recently, the role of Piezo1 channel in apoptosis has been investigated and its implications for pathophysiology have been also reported (41).
At the steady state, Piezo1 functions to ensure cellular Ca2+ homeostasis, preventing p53-dependent senescence and apoptosis (42). Silencing of Piezo1 provoked compensatory upregulation of T-type voltage-gated Ca2+ channels, namely CaV3.1 and CaV3.2, in skeletal muscle stem cells. This led to Ca2+-dependent activation of conventional protein kinase C (cPKC) to promote the expression of NOX4, a reactive oxygen species (ROS)-generating enzyme, causing p53-dependent senescence and apoptosis in Piezo1-deficient skeletal muscle stem cells. On the other hand, cells under abnormal mechanical stresses show increased expression and activation of Piezo1, thereby allowing substantial Ca2+ influx, which consequently leads to cytotoxicity. In human articular chondrocytes constantly exposed to hydrostatic pressure, the increase in intracellular Ca2+ levels was accompanied by the upregulated expression of p53 and cleaved caspase-3 and -9, which was inhibited upon the treatment with gadolinium (Gd3+) or an siRNA for Piezo1 (17). Similarly, a recent study suggested that pancreatitis can be caused by chronic death of pancreatic acinar cells due to Piezo1-mediated Ca2+ overload following pressure on the gland (11). In mice, mechanical pressure within the gland led to the development of pancreatitis; however, the mice with pancreatic acinar cell-specific deletion of Piezo1 were protected from this. In addition, it was demonstrated that administration of Yoda1, a Piezo1 agonist, directly induced pancreatic acinar cell death. Hence, this study may explain why pancreatitis develops in humans after abdominal trauma, pancreatic duct obstruction, pancreatography, or pancreatic surgery, which can exert pressure on the gland.
Mechano-physical properties of the microenvironment in which cells reside can also control the activity of Piezo1 channel. Among others, stiffness is one of the most studied and critical physical features of various tissues. On a stiff substrate with high elastic modulus (25 kPa), human nucleus pulposus (NP) cells showed upregulated expression of MS Piezo1 channel as well as elevated levels of intracellular Ca2+ when compared to the cells on a soft substrate (1 kPa) (31). Excessive intracellular Ca2+ levels brought about an increase in the ROS levels and expression of ER stress markers, GRP78 and CHOP, subsequently leading to senescence and apoptosis in Piezo1-activated NP cells on the stiff substrate. In a rat model of intervertebral disc degeneration, which is known to be developed by excessive mechanical loading on NP tissues, siRNA-mediated inhibition of Piezo1 effectively ameliorated the progression of intervertebral disc degeneration by reducing the elastic modulus of NP tissues and apoptotic cell population (31). Notably, a mechanical compression of human NP cells yielded similar results; more NP cells expressed Piezo1 and underwent cellular senescence or apoptosis when the extent of compression was higher or longer (43).
Taken together, increase in intracellular Ca2+ levels to the extent of cytotoxicity through the extensive activation of Piezo1 is a key step in initiating mitochondrial apoptotic pathway in the presence of mechanical stimuli, such as compression and substrate stiffness, which induce membrane displacement (Fig. 1). Thus, inhibition of Piezo1 or removal of abnormal mechanical stimulation are potentially therapeutic options for apoptosis-associated diseases.
Fig. 1.
Piezo1-mediated Ca2+-dependent apoptosis mechanism. Piezo1, a mechanosensitive ion channel, opens in response to mechanical (tensile, compressive, shear) forces and physical cues in the extracellular matrix (ECM) that induce membrane tension, allowing Ca2+ to enter the cytosol. Mitochondrial damage caused by Ca2+ overload increases the levels of reactive oxygen species (ROS) that cause endoplasmic reticulum (ER) stress, indicated by upregulation of GRP78 and CHOP. Ca2+ is then released from the stressed ER via ryanodine receptors (RyRs) and inositol-1,4,5-trisphosphate receptors (Ins(1,4,5)P3Rs) and taken up by mitochondria via mitochondrial calcium uniporters (MCU). In addition, cytosolic Ca2+ activates the calpain protease to cleave and translocate p53 and Bcl-2-associated X (BAX) proteins to the mitochondria. Once Ca2+ binds to cardiolipin at the inner mitochondrial membrane, cytochrome c (Cyt c) is dissociated from cardiolipin and the BAX protein weakens mitochondrial outer membrane integrity, then releases Cyt c into the cytosol through the mitochondrial permeability transition pore (MPTP). Subsequently, Cyt c forms an apoptosome complex with apoptotic protease activating factor-1 (APAF-1) and procaspase-9 to promote the activation of caspase-9 and caspase-3 to initiate apoptosis. Piezo1-mediated apoptosis under mechanical stress can be inhibited by treatment with Gd3+ (an inhibitor of mechanically gated cation channels), GsMTx4 (a peptide blocker of Piezo1), or RNA interference (RNAi) to Piezo1, whereas the Piezo1 agonist Yoda1 promotes Piezo1-induced apoptosis by lowering the mechanical activation threshold of Piezo1.
THE ROLE OF PIEZO1 IN FERROPTOSIS
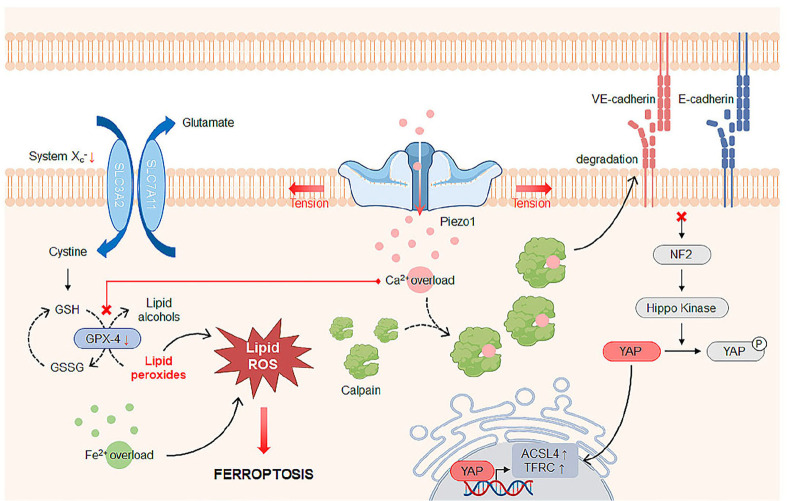
Ferroptosis is an iron-dependent form of programmed cell death characterized by the accumulation of lipid peroxides and unique morphological features (44). Under a microscope, ferroptotic cells show obvious mitochondrial shrinkage with increased mitochondrial membrane densities and reduced mitochondrial crista, mitochondrial outer membrane rupture, intact plasma membrane, and normal nucleus (45). The mechanisms underlying ferroptosis involve reduced intracellular glutathione (GSH) levels and decreased activity of glutathione peroxidase-4 (GPX-4), an enzyme that metabolizes lipid peroxides into non-toxic lipid alcohols (44). Otherwise, Fe2+ oxidizes lipids in a Fenton-like manner, resulting in a large amount of ROS, thereby promoting ferroptosis, which can be prevented by iron chelators (46). Several mechanisms have been proposed for how ferroptosis is induced, including the calcium-dependent mechanisms. Adding extracellular glutamate induces GSH depletion in cells by blocking the glutamate-cystine antiporter system Xc-(transporter subunit is encoded by the SLC7A11 gene), eventually resulting in ferroptosis. Henke et al. (47) observed an increase in extracellular Ca2+ influx in cells undergoing extracellular glutamate-induced ferroptosis. Inappropriate Ca2+ influx resulted due to a significant induction of store-operated calcium entry caused by the upregulation of a selective Ca2+ channel, namely the calcium release-activated calcium channel protein 1 (ORAI1).
Recent studies have indicated that Piezo1 as a MS Ca2+ channel also plays an important role in ferroptosis. As GPX-4 acts as a gatekeeper against ferroptosis, it is suppressed in ferroptosis and often used as a marker for its evaluation. When subjected to mechanical loading concentrated in knee joints by destabilizing the medial meniscus, chondrocyte-specific Gpx-4 knockout mice developed more severe osteoarthritis compared with their wild-type littermates (48). Mechanical loading on human cartilage and mouse chondrocytes increased Piezo1 expression and triggered GPX-4-mediated ferroptosis, which was attenuated upon the suppression of Piezo1 activity using GsMTx4, a selective inhibitor of cationic MS channels, or preventing extracellular Ca2+ influx, thereby eventually reducing the severity of osteoarthritis. However, treatment with GsMTx4 was ineffective in terms of reversing the mechanical overload-induced chondrocyte damage in Gpx-4 knockout mice, hinting at Piezo1 induced ferroptosis via GPX-4 inhibition.
Ionizing radiations induce lung injury by destroying pulmonary endothelial cell (PEC) functions (49). Recently, it was reported that PECs exposed to ionizing radiations underwent ferroptosis (49). In accordance with the finding that Piezo1 expression was increased in PECs after the exposure to ionizing radiations, Yoda1 treatment expanded ferroptotic cell population among non-radiated PECs while treatment with GsMTx4 reduced the number of ferroptotic cells among the radiated PECs. Mechanistically, it was demonstrated that elevated intracellular Ca2+ levels increased calpain protease activity, which degraded VE-cadherin to promote ferroptosis. Similarly, it was reported that E-cadherin inhibited ferroptosis by activating the NF2-Hippo pathway, which suppresses pro-ferroptotic YAP activation in epithelial cells (50).
Taken together, the role of Piezo1 in ferroptosis depends on the regulation of GPX-4 (Fig. 2); future studies are required to analyze how Piezo1 controls the GPX-4 activity and whether Ca2+ ions are involved in the regulation of GPX-4.
Fig. 2.
Piezo1-mediated Ca2+-dependent ferroptosis mechanism. Piezo1 is activated upon mechanical stimulation and increases the intracellular Ca2+ level, increasing the activity of the calpain protease that degrades (V)E-cadherin. Cells with loss of (V)E-cadherin and subsequent inactivation of NF2 and hippo kinase are susceptible to ferroptosis due to upregulation of multiple regulators of ferroptosis including ACSL4 and TFRC through YAP activation. The cystine/glutamate antiporter system Xc-(consisting of light chain SLC7A11 and heavy chain SLC3A2) mediates uptake of cystine for the production of cysteine and glutathione (GSH), which is converted to glutathione disulfide (GSSG) by glutathione peroxidase-4 (GPX-4), an antioxidant enzyme that metabolizes lipid peroxides into non-toxic lipid alcohols. However, Ca2+ suppresses the expression of GPX-4, which promotes accumulation of lipid peroxides that increase lipid ROS, consequently causing ferroptosis. Otherwise, Fe2+ binds to reactive oxygen species (ROS) to participate in iron-dependent lipid peroxidation and finally induce ferroptosis. Abbreviations: ACSL4, Acyl-CoA Synthetase Long Chain Family Member 4; TFRC, Transferrin Receptor 1.
POTENTIAL OF TARGETING MECHANOSENSITIVE ION CHANNELS FOR CANCER TREATMENT
Cancer cells acquire resistance to apoptosis due to their heterogeneity and mutations, leading to malignant progression and recurrence after anti-cancer therapies. Therefore, selective induction of apoptosis in cancer cells is of great importance for cancer treatment. As over- or extended activation of MS cation channels disrupts Ca2+ homeostasis, often causing cell deaths, targeting MS cation channels is a promising strategy for killing cancer cells. In early 2010, Kim et al. (51) developed disc-shaped magnetic microparticles (approximately 1 μm in diameter), which oscillate under an alternating magnetic field and transmit a cytotoxic level of mechanical force to cancer cells. Thus, interfacing with these microdiscs disturbed membrane integrity and increased intracellular Ca2+ levels, possibly through the activation of MS ion channels, which eventually induced programmed death in cancer cells (Fig. 3A). These microdiscs can be biofunctionalized with antibodies capturing cancer cell surface markers to enhance target specificity; they can further be scaled down to nanodiscs (approximately 100 nm in diameter).
Fig. 3.
Cancer treatment strategies through the induction of mechanosensitive ion channel-mediated Ca2+-dependent cell death by mechanical perturbations. (A) Microdiscs biofunctionalized with an antibody (Ab) that captures cancer cell surface marker vibrate (i.e., oscillation) under a magnetic field to deliver cytotoxic levels of mechanical force to cancer cells. Interfacing with these microdiscs disrupts membrane integrity and increases intracellular Ca2+ levels, presumably through activation of mechanosensitive (MS) ion channels, eventually leading to programmed cell death in cancer cells. (B) Schematic diagram highlighting the different expression of mechanosensing proteins including Piezo1, tropomyosin 2.1 (Tpm2.1) and myosin IIA between normal and cancer cells. Cancer cells have higher levels of Piezo1 than normal cells, making them more susceptible to apoptosis due to the increase in intracellular Ca2+ levels to the extent of cytotoxicity upon mechanical stimulation. Cyclic stretching stimulates normal cell growth while slowing growth and eventually causing apoptosis in cancer cells. Stretch-induced apoptosis in cancer cells occurs through the Piezo1-mediated Ca2+-dependent mitochondrial apoptosis pathway. Tpm2.1 is eliminated from cancer cells, allowing them to grow anchorage-independently. Cancer cells recovered with Tpm2.1 survive and proliferate upon cyclic stretching, whereas normal cells depleted of Tpm2.1 become susceptible to stretch-induced apoptosis. In addition, restoration of myosin IIA inhibits cancer cell growth, which can induce apoptosis through induction of Piezo1 activation (see Fig. 3C). (C) Piezo1 is activated by ultrasound (US)-mediated mechanical force, triggering Ca2+ influx to activate calpain proteases, which activates the mitochondrial apoptosis pathway and induces microtubule disassembly. Destabilized microtubules enhance myosin IIA contractility through activation of GEF-H1, a Rho guanine nucleotide exchange factor that can increase RhoA activity. Increased contractility, in turn, promotes Piezo1 expression and localization to peripheral adhesions where Piezo1 channel opening allows Ca2+ influx as a positive feedback loop. (D) Cancer cells in contact with nanogels carrying the MscL plasmid and polyethylenamine (PEI) as a transfection reagent are induced to express and activate MscL MS ion channels upon mechanical US waves, resulting in sustained Ca2+ entry to cause apoptosis when ultrasound was applied. Apoptotic cell fragments act as tumor-associated antigens to promote dendritic cell (DC) maturation and subsequent anti-cancer CD8+ T cell activation. Schematic illustrations were recomposed based on key finding in refs. (51) Kim et al. Nat Mater (2010) for (A), (54) Tijore et al. Biomaterials (2021) for (B), (61) Singh et al. Bioeng Transl Med (2021) for (C) and (65) He et al. Chem Eng J (2021) for (D).
To overcome the reduced sensitivity of cancer cells toward apoptosis, Wen et al. (52) induced non-apoptotic cell death in cancer cells by introducing a gain-of-function mutation of MscL. The gain-of-function mutant V23A-MscL constitutively opened, thus enabling intracellular Ca2+ overload, which resulted in non-apoptotic cell death characterized by cytoplasmic vacuolization in human hepatocellular carcinoma cells transduced with V23A-MscL; it also suppressed the tumor growth in a xenograft model of human hepatocellular carcinoma in mice. Notably, mutant G26C-MscL, which opened in response to [2-(trimethylammonium)ethyl] methane thiosulfonate bromide (MTSET), induced necrotic cell death with distinct morphological features such as disruption and aggregation of ER, membrane leakage following organelle-free membrane blebbing, impaired nuclei, and dispersed chromatin followed by clearing of the cytosol and plasma membrane rupture (52).
Recent studies have shown that cancer cells with higher sensitivity to mechanical stresses than normal cells undergo Ca2+-dependent apoptosis upon mechanical perturbations. This distinct feature of cancer cells can be leveraged for their selective killing with minimal effect on normal cells by applying mechanical stresses via stretching or ultrasound.
Vulnerability of cancer cells to mechanical stress
Fundamental differences exist between normal and cancer cells, including biochemical signaling, metabolism, and anchorage (in)dependence. Besides, it has been proven that cancer cells also differ from normal cells in terms of mechanosensing, which influences cell fate under different mechanical stresses. To evaluate whether stretching reduces the tumor growth, primary mouse mammary tumor cells were implanted within third mammary fat pad tissues either subjected to stretching or non-stretched (53). Regular stretching markedly diminished tumor volume in mice compared to the non-stretched mice. Remarkably, with the significant upregulation of specialized pro-resolving lipid mediators (SPMs) RvD1 and RvD2, the proportion of IL-2+ CD8+ cytotoxic T cells increased while that of PD-1+ CD8+ exhausted T cell population decreased in the stretched mice, indicating an improvement in cytotoxic immune responses upon stretching. More directly, Tijore et al. (54) revealed that cyclic stretching lowered the growth rate and finally caused apoptosis in cancer cells, whereas it stimulated the growth of normal cells. The stretch-induced apoptosis occurred in cancer cells through Piezo1-mediated Ca2+ influx that activates calpain-2, leading to the cleavage and translocation of Bcl-2-accociated X (BAX) proteins into mitochondria where they weaken mitochondrial outer membrane integrity followed by the leakage of Cyt c into the cytosol to activate caspase-3 (Fig. 3B). Interestingly, tropomyosin 2.1 (Tpm2.1)-restored cancer cells survived and proliferated upon cyclic stretching, while Tpm2.1-depleted normal cells were vulnerable to stretch-induced apoptosis. Tpm2.1 was previously shown as a rigidity sensing protein that disappears in malignantly transformed cells, enabling them to grow on soft matrices (i.e., anchorage-independent growth or anoikis resistance) (55). These findings indicate that proteins involved in mechanosensing are critical for cell survival.
Non-invasive induction of cancer cell death using ultrasound
More recently, ultrasound has been used for non-invasive cancer treatment. Tijore et al. (56) have reported that cancer cells, but not normal cells, undergo Ca2+-dependent apoptosis through Piezo1 channel by activating a calpain-dependent mitochondrial apoptosis pathway in response to mechanical perturbation by low-frequency ultrasound. The Piezo1 channels on pancreatic ductal adenocarcinoma (PDAC) cells were gated by ultrasound with microbubbles that generated mechanical forces via cavitation, which triggered mitochondrial BAX-Cyt c apoptosis pathway, possibly through the activation of Ca2+-dependent calpain proteases (57). The anti-cancer effect of ultrasound with microbubbles was reduced in Piezo1-knockdown PDAC xenograft models. The involvement of Piezo1 in ultrasound-mediated cancer cell apoptosis may also explain how the application of ultrasound with microbubbles was therapeutically effective in other types of cancers, including breast, colon, and liver cancers (57-60).
Combination of ultrasound and chemotherapeutic drugs is synergistic in treating cancer. Singh et al. (61) demonstrated that ultrasound-mediated mechanical forces disrupt microtubules via the activation of calpain proteases. Destabilized microtubules enhanced myosin IIA contractility through activation of GEF-H1, a Rho guanine nucleotide exchange factor that can increase RhoA activity. Consistently, myosin IIA was reported as a rigidity sensing protein, and its restoration inhibited cancer cell growth (55). Increased contractility, in turn, promoted Piezo1 expression and localization to the peripheral adhesions where opening of Piezo1 channel allowed Ca2+ influx as a feedback loop (Fig. 3C), suggesting that ultrasound can enhance cancer cell death when combined with microtubule destabilizing agents (61).
Indeed, mechanical induction of Ca2+-dependent apoptosis in cancer cells relies on higher expression of Piezo1 in cancer cells than in normal cells (Fig. 3B). Piezo1 is highly expressed in human PDAC and gastric cancer cells and tissues (57, 62). Inhibiting Piezo1 decreased the viability of cancer cells, while activation of Piezo1 with Yoda1 at optimal concentration promoted colony formation (62), thereby suggesting the presence of distinct control mechanisms of Ca2+ signaling between normal and malignant cells (63). However, higher concentration of Yoda1 led to the apoptosis of cancer cells, which might be due to extreme Ca2+ levels (62). Supportively, metastatic malignant cells with a lower expression of Piezo1 channel than primary cancer cells were resistant to Piezo1-mediated apoptosis when exposed to fluid shear stress; this is possibly because metastatic cells must survive aberrant fluid shear stress in the circulation (64).
Hence, Ca2+-dependent apoptosis can be achieved by introducing ectopic MS ion channels in cancer cell. He et al. (65) adopted a sonogenetic nanosystem, which was consisted of iron alginate nanogel as a carrier, MscL plasmids and polyethylenamine (PEI) as a transfection reagent; upon exposure to ultrasound, the MscL MS ion channel was specifically expressed and activated in nanogel-contacted cells. In cancer cells treated with this sonogenetic nanosystem, MscL was expressed on cell surfaces and was activated to allow continuous Ca2+ entry, causing apoptosis when ultrasound was applied. Furthermore, in mice inoculated with mouse melanoma cells, ultrasound triggered not only melanoma cell apoptosis but also anti-cancer immune responses; this was because apoptotic cell fragments acted as tumor-associated antigens to promote dendritic cell maturation and subsequent CD8+ T cell activation (Fig. 3D).
CONCLUSION
Programmed cell death due to intracellular Ca2+ overload after exposure to mechanical stimuli is called “mechanoptosis”, which is regulated by MS ion channels such as Piezo1 (61). Overexpression or hyperactivation of Piezo1 induces uncontrolled Ca2+-dependent cell deaths, including apoptosis and ferroptosis, causing a plethora of human diseases. Hence, Piezo1 is promising as a therapeutic target. For example, Piezo1 blockade using RNA interference or a peptide inhibitor (e.g., GsMTx4) may relieve pancreatitis and osteoarthritis by preventing Ca2+-dependent apoptosis/ferroptosis of pancreatic acinar cells and chondrocytes, respectively. However, the therapeutic potential of Piezo1 inhibition in other human disorders, such as developmental disorders, immunodeficiency, autoimmune diseases, and neurodegeneration, remains to be investigated.
On the other hand, cancer cells are intrinsically resistant to programmed cell death because they differ from normal cells in terms of expression of mechanosensing proteins, including Tpm2.1, myosin IIA, and Piezo1. Because the basal levels of Piezo1 expression are higher in most cancer cells than those in normal cells, the induction of Piezo1 may have potential anti-cancer therapeutic effects. Considering this, Piezo1 agonist (e.g., Yoda1) or mechanical stimulation (e.g., stretch or ultrasound) has been applied to cause Ca2+-dependent cancer cell death, while promoting the ability of the normal cells to regenerate. In conclusion, information pertaining to Piezo1 expressional levels and subcellular localization at different developmental and/or environmental stages will help in developing therapeutic strategy against cancer. Advances in biomedical engineering would assist in developing anti-cancer devices that locally generate a mechanical stimulus as well as in designing cancer-specific induction of Piezo1-mediated genetic circuit upon mechanical perturbations.
Funding Statement
ACKNOWLEDGEMENTS The present research was supported by the research fund of Dankook University in 2020.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
References
- 1.Barnes JM, Przybyla L, Weaver VM. Tissue mechanics regulate brain development, homeostasis and disease. J Cell Sci. 2017;130:71–82. doi: 10.1242/jcs.191742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Su SA, Li W, et al. Piezo1-mediated mechanotransduction promotes cardiac hypertrophy by impairing calcium homeostasis to activate calpain/calcineurin signaling. Hypertension. 2021;78:647–660. doi: 10.1161/HYPERTENSIONAHA.121.17177. [DOI] [PubMed] [Google Scholar]
- 3.Bonakdar N, Gerum R, Kuhn M, et al. Mechanical plasticity of cells. Nat Mater. 2016;15:1090–1094. doi: 10.1038/nmat4689. [DOI] [PubMed] [Google Scholar]
- 4.Rafiq NBM, Nishimura Y, Plotnikov SV, et al. A mechano-signalling network linking microtubules, myosin IIA filaments and integrin-based adhesions. Nat Mater. 2019;18:638–649. doi: 10.1038/s41563-019-0371-y. [DOI] [PubMed] [Google Scholar]
- 5.Sanghvi-Shah R, Weber GF. Intermediate filaments at the junction of mechanotransduction, migration, and development. Front Cell Dev Biol. 2017;5:81. doi: 10.3389/fcell.2017.00081.2b040d4f48e4405a890b6cdf32e0a66c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseini K, Sbosny L, Poser I, Fischer-Friedrich E. Binding dynamics of alpha-actinin-4 in dependence of actin cortex tension. Biophys J. 2020;119:1091–1107. doi: 10.1016/j.bpj.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin P, Jan LY, Jan YN. Mechanosensitive ion channels: structural features relevant to mechanotransduction mechanisms. Annu Rev Neurosci. 2020;43:207–229. doi: 10.1146/annurev-neuro-070918-050509. [DOI] [PubMed] [Google Scholar]
- 8.Chery DR, Han B, Li Q, et al. Early changes in cartilage pericellular matrix micromechanobiology portend the onset of post-traumatic osteoarthritis. Acta Biomater. 2020;111:267–278. doi: 10.1016/j.actbio.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao W, Hasan H, Anderson DE, Lee W. The role of mechanically-activated ion channels Piezo1, Piezo2, and TRPV4 in chondrocyte mechanotransduction and mechano-therapeutics for osteoarthritis. Front Cell Dev Biol. 2022;10:885224. doi: 10.3389/fcell.2022.885224.57fe20ee6d95468e97d72a6df018270f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunzelmann K. Ion channels in regulated cell death. Cell Mol Life Sci. 2016;73:2387–2403. doi: 10.1007/s00018-016-2208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romac JM, Shahid RA, Swain SM, Vigna SR, Liddle RA. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat Commun. 2018;9:1715. doi: 10.1038/s41467-018-04194-9.10e1a87d36af415ca7fb7de77dc16ef2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao J, Lu W, Chen Y, et al. Upregulation of Piezo1 (Piezo type mechanosensitive ion channel component 1) enhances the intracellular free calcium in pulmonary arterial smooth muscle cells from idiopathic pulmonary arterial hypertension patients. Hypertension. 2021;77:1974–1989. doi: 10.1161/HYPERTENSIONAHA.120.16629. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez-Barrantes R, Cordova C, Gatica S, et al. Transient receptor potential vanilloid 1 expression mediates capsaicin-induced cell death. Front Physiol. 2018;9:682. doi: 10.3389/fphys.2018.00682.2b847890b25b4c07b72f2f2fa1cb78b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kearney EM, Prendergast PJ, Campbell VA. Mechanisms of strain-mediated mesenchymal stem cell apoptosis. J Biomech Eng. 2008;130:061004. doi: 10.1115/1.2979870. [DOI] [PubMed] [Google Scholar]
- 15.Hutcheson JD, Venkataraman R, Baudenbacher FJ, Merryman WD. Intracellular Ca2+ accumulation is strain-dependent and correlates with apoptosis in aortic valve fibroblasts. J Biomech. 2012;45:888–894. doi: 10.1016/j.jbiomech.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casas S, Novials A, Reimann F, Gomis R, Gribble FM. Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia. 2008;51:2252–2262. doi: 10.1007/s00125-008-1111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence KM, Jones RC, Jackson TR, et al. Chondroprotection by urocortin involves blockade of the mechanosensitive ion channel Piezo1. Sci Rep. 2017;7:5147. doi: 10.1038/s41598-017-04367-4.57888e6763164f2e881cc57bb81d7732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez-Berdugo D, Rofes L, Arreola V, Martin A, Molina L, Clave P. A comparative study on the therapeutic effect of TRPV1, TRPA1, and TRPM8 agonists on swallowing dysfunction associated with aging and neurological diseases. Neurogastroenterol Motil. 2018;30:e13185. doi: 10.1111/nmo.13185. [DOI] [PubMed] [Google Scholar]
- 20.Romero LO, Massey AE, Mata-Daboin AD, et al. Dietary fatty acids fine-tune Piezo1 mechanical response. Nat Commun. 2019;10:1200. doi: 10.1038/s41467-019-09055-7.79b47773086f40d2b56953992c83ae64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leisengang S, Ott D, Murgott J, et al. Effects of gabapentinoids on responses of primary cultures from rat dorsal root ganglia to inflammatory or somatosensory stimuli. J Basic Clin Physiol Pharmacol. 2020;31:0261. doi: 10.1515/jbcpp-2019-0261. [DOI] [PubMed] [Google Scholar]
- 22.Rajeshwar TR, Anishkin A, Sukharev S, Vanegas JM. Mechanical activation of mscl revealed by a locally distributed tension molecular dynamics approach. Biophys J. 2021;120:232–242. doi: 10.1016/j.bpj.2020.11.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinac B. 2021 Nobel Prize for mechanosensory transduction. Biophys Rev. 2022;14:15–20. doi: 10.1007/s12551-022-00935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coste B, Mathur J, Schmidt M, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis AH, Grandl J. Inactivation kinetics and mechanical gating of Piezo1 ion channels depend on subdomains within the cap. Cell Rep. 2020;30:870–880. doi: 10.1016/j.celrep.2019.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coste B, Xiao B, Santos JS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YC, Guo YR, Miyagi A, Levring J, MacKinnon R, Scheuring S. Force-induced conformational changes in PIEZO1. Nature. 2019;573:230–234. doi: 10.1038/s41586-019-1499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao H, Zhu M, Lau K, et al. Oscillatory cortical forces promote three dimensional cell intercalations that shape the murine mandibular arch. Nat Commun. 2019;10:1703. doi: 10.1038/s41467-019-09540-z.a1919169bcc7429c80847ae4da278f58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jetta D, Bahrani Fard MR, Sachs F, Munechika K, Hua SZ. Adherent cell remodeling on micropatterns is modulated by Piezo1 channels. Sci Rep. 2021;11:5088. doi: 10.1038/s41598-021-84427-y.6f4a2c6f55c84bee8e66b8d6b47449e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Ke W, Wang K, et al. Mechanosensitive ion channel Piezo1 activated by matrix stiffness regulates oxidative stress-induced senescence and apoptosis in human intervertebral disc degeneration. Oxid Med Cell Longev. 2021;2021:8884922. doi: 10.1155/2021/8884922.0deac54124714deda71526e6d5166b0d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Hou L, Li F, et al. Piezo1-mediated mechanosensation in bone marrow macrophages promotes vascular niche regeneration after irradiation injury. Theranostics. 2022;12:1621–1638. doi: 10.7150/thno.64963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sforna L, Michelucci A, Morena F, et al. Piezo1 controls cell volume and migration by modulating swelling-activated chloride current through Ca2+ influx. J Cell Physiol. 2022;237:1857–1870. doi: 10.1002/jcp.30656. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa K, Fujii S, Matsumoto S, Tajiri Y, Kikuchi A, Kiyoshima T. YAP signaling induces PIEZO1 to promote oral squamous cell carcinoma cell proliferation. J Pathol. 2021;253:80–93. doi: 10.1002/path.5553. [DOI] [PubMed] [Google Scholar]
- 35.Li XF, Zhang Z, Chen ZK, Cui ZW, Zhang HN. Piezo1 protein induces the apoptosis of human osteoarthritis-derived chondrocytes by activating caspase-12, the signaling marker of ER stress. Int J Mol Med. 2017;40:845–853. doi: 10.3892/ijmm.2017.3075. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 38.Dubois C, Vanden Abeele F, Prevarskaya N. Targeting apoptosis by the remodelling of calcium-transporting proteins in cancerogenesis. FEBS J. 2013;280:5500–5510. doi: 10.1111/febs.12246. [DOI] [PubMed] [Google Scholar]
- 39.Hajnoczky G, Csordas G, Das S, et al. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baughman JM, Perocchi F, Girgis HS, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang GP, Xu J, Cao LL, et al. Piezo1 induced apoptosis of type II pneumocytes during ARDS. Respir Res. 2019;20:118. doi: 10.1186/s12931-019-1083-1.ee2e668e7e014b559108c7957750ae7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng Y, Du J, Gunther S, et al. Mechano-signaling via Piezo1 prevents activation and p53-mediated senescence of muscle stem cells. Redox Biol. 2022;52:102309. doi: 10.1016/j.redox.2022.102309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi S, Kang XJ, Zhou Z, He ZM, Zheng S, He SS. Excessive mechanical stress-induced intervertebral disc degeneration is related to Piezo1 overexpression triggering the imbalance of autophagy/apoptosis in human nucleus pulpous. Arthritis Res Ther. 2022;24:119. doi: 10.1186/s13075-022-02804-y.2fcdad797ab74230bb4f0862acc20225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z, Chen L, Chen C, et al. Targeting ferroptosis in breast cancer. Biomark Res. 2020;8:58. doi: 10.1186/s40364-020-00230-3.8219346f7b8e4a108a12c99b2b348615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henke N, Albrecht P, Bouchachia I, et al. The plasma membrane channel ORAI1 mediates detrimental calcium influx caused by endogenous oxidative stress. Cell Death Dis. 2013;4:e470. doi: 10.1038/cddis.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S, Li W, Zhang P, et al. Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx. J Adv Res. 2022;41:63–75. doi: 10.1016/j.jare.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo XW, Zhang H, Huang JQ, et al. PIEZO1 ion channel mediates ionizing radiation-induced pulmonary endothelial cell ferroptosis via Ca2+/Calpain/VE-Cadherin signaling. Front Mol Biosci. 2021;8:725274. doi: 10.3389/fmolb.2021.725274.6e2017851802487e855f6610c9360f07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J, Minikes AM, Gao M, et al. Publisher correction: intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:E20. doi: 10.1038/s41586-019-1480-0. [DOI] [PubMed] [Google Scholar]
- 51.Kim DH, Rozhkova EA, Ulasov IV, et al. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat Mater. 2010;9:165–171. doi: 10.1038/nmat2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wen X, Tang S, Hong F, et al. Non-apoptotic cell death induced by opening the large conductance mechanosensitive channel MscL in hepatocellular carcinoma HepG2 cells. Biomaterials. 2020;250:120061. doi: 10.1016/j.biomaterials.2020.120061. [DOI] [PubMed] [Google Scholar]
- 53.Berrueta L, Bergholz J, Munoz D, et al. Stretching reduces tumor growth in a mouse breast cancer model. Sci Rep. 2018;8:7864. doi: 10.1038/s41598-018-26198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tijore A, Yao M, Wang YH, et al. Selective killing of transformed cells by mechanical stretch. Biomaterials. 2021;275:120866. doi: 10.1016/j.biomaterials.2021.120866. [DOI] [PubMed] [Google Scholar]
- 55.Yang B, Wolfenson H, Chung VY, et al. Stopping transformed cancer cell growth by rigidity sensing. Nat Mater. 2020;19:239–250. doi: 10.1038/s41563-019-0507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tijore A, Margadant F, Yao M, et al. Ultrasound-mediated mechanical forces selectively kill tumor cells. bioRxiv. 2020:332726. doi: 10.1101/2020.10.09.332726. [DOI] [Google Scholar]
- 57.Song Y, Chen J, Zhang C, et al. Mechanosensitive channel Piezo1 induces cell apoptosis in pancreatic cancer by ultrasound with microbubbles. iScience. 2022;25:103733. doi: 10.1016/j.isci.2022.103733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang P, You X, Pan M, et al. A novel therapeutic strategy using ultrasound mediated microbubbles destruction to treat colon cancer in a mouse model. Cancer Lett. 2013;335:183–190. doi: 10.1016/j.canlet.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Wang P, Chen X, et al. Activation of microbubbles by low-intensity pulsed ultrasound enhances the cytotoxicity of curcumin involving apoptosis induction and cell motility inhibition in human breast cancer MDA-MB-231 cells. Ultrason Sonochem. 2016;33:26–36. doi: 10.1016/j.ultsonch.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Zhang C, Huang P, Zhang Y, et al. Anti-tumor efficacy of ultrasonic cavitation is potentiated by concurrent delivery of anti-angiogenic drug in colon cancer. Cancer Lett. 2014;347:105–113. doi: 10.1016/j.canlet.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 61.Singh A, Tijore A, Margadant F, et al. Enhanced tumor cell killing by ultrasound after microtubule depolymerization. Bioeng Transl Med. 2021;6:e10233. doi: 10.1002/btm2.10233.07ed7e290f1c4424b79a309f33d9ab36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Cheng G, Miao Y, et al. Piezo type mechanosensitive ion channel component 1 facilitates gastric cancer omentum metastasis. J Cell Mol Med. 2021;25:2238–2253. doi: 10.1111/jcmm.16217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marchi S, Giorgi C, Galluzzi L, Pinton P. Ca2+ fluxes and cancer. Mol Cell. 2020;78:1055–1069. doi: 10.1016/j.molcel.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 64.Greenlee JD, Liu K, Lopez-Cavestany M, King MR. Piezo1 mechano-activation is augmented by resveratrol and differs between colorectal cancer cells of primary and metastatic origin. Molecules. 2022;27:5430. doi: 10.3390/molecules27175430.f6873157261e43cebcd5fe425486f218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He T, Wang H, Wang T, et al. Sonogenetic nanosystem activated mechanosensitive ion channel to induce cell apoptosis for cancer immunotherapy. Chem Eng J. 2021;407:127173. doi: 10.1016/j.cej.2020.127173. [DOI] [Google Scholar]