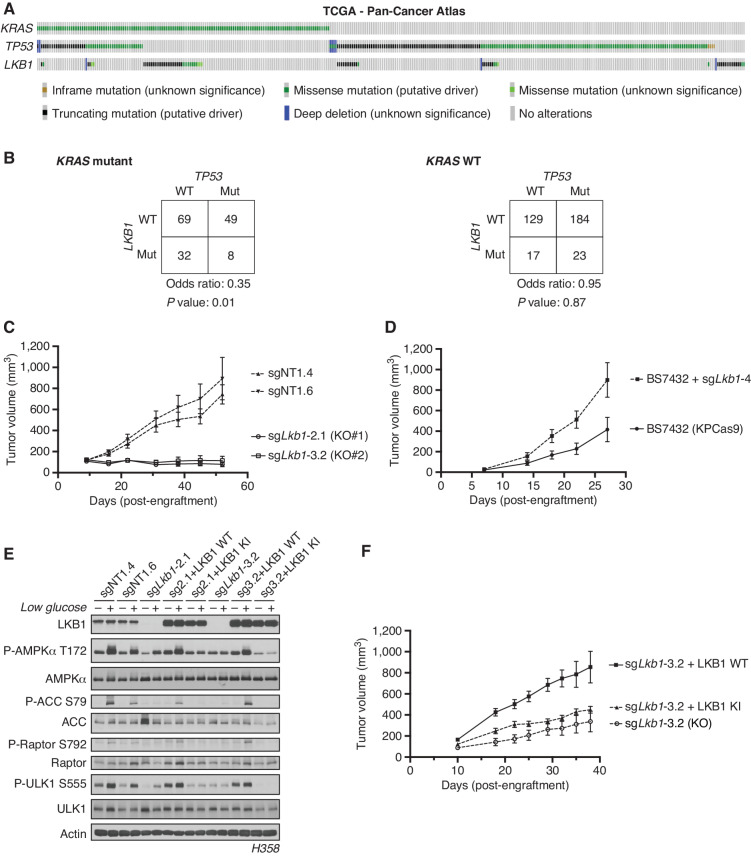
Figure 1.
Co-occurrence of KRAS, TP53, and LKB1 mutations differentially affects the growth of human and mouse LUADs. A, The TCGA Pan-Cancer Atlas oncoprint of co-occurrence of KRAS, TP53, and LKB1 in hLUAD patients. B, Fisher exact test of the statistical likelihood of co-occurrence of LKB1 and TP53 mutations in a KRAS-mutant or wild-type (WT) background, respectively. C, Graph of mean (± SEM) tumor volumes of subcutaneous flank injections of H358 (KRAS;TP53) isogenic clones expressing Cas9 and a nontargeting (sgNT1.4 and sgNT1.6) or LKB1-specific (sgLkb1-2.1 and sgLkb1-3.2) guide RNA; 1 × 106 cells were implanted in the right hind flank (n = 10 per cohort). KO, knockout. D, Mean (± SEM) volumes of isogenic KPCas9 LUAD allograft tumors expressing a nontargeting (BS7432) or Lkb1-specific (sgLkb1-4) guide RNA; 0.25 × 106 cells were implanted in left and right flank of C57Bl/6J mice (n = 10 per cohort). E, Western blot analysis of H358 (KRAS;TP53) isogenic clones (KP: sgNT1.4 and sgNT1.6; KPL: sgLkb1-2.1 and sgLkb1-3.2) and KPL lines with additional transgenic expression of guide RNA resistant LKB1 WT (sgLkb1-2.1 + LKB1 WT and sgLkb1-3.2 + LKB1 WT) or LKB1 kinase-inactive (KI; sgLkb1-2.1 + LKB1 KI and sgLkb1-3.2 LKB1 KI) and treated with 11.1 mmol/L or 0.5 mmol/L glucose for 6 hours as indicated. Restoration of AMPK signaling in LKB1 WT lines in response to 0.5 mmol/L glucose validated by blotting for P-AMPK Thr172 and downstream substrates (P-ACC S79, P-ULK1 S555, and P-Raptor S792). Similar results were observed in three independent experiments and in an additional KRAS;TP53 cell line, H2009 (Supplementary Fig. S1E). F, Graph of mean (± SEM) tumor volumes of subcutaneous flank injections of H358 (KRAS;TP53) isogenic clones with transgenic expression of an empty vector (KO) or guide RNA resistant LKB1 WT or LKB1 KI; 1 × 106 cells were implanted in the right hind flank (n = 10 per cohort).