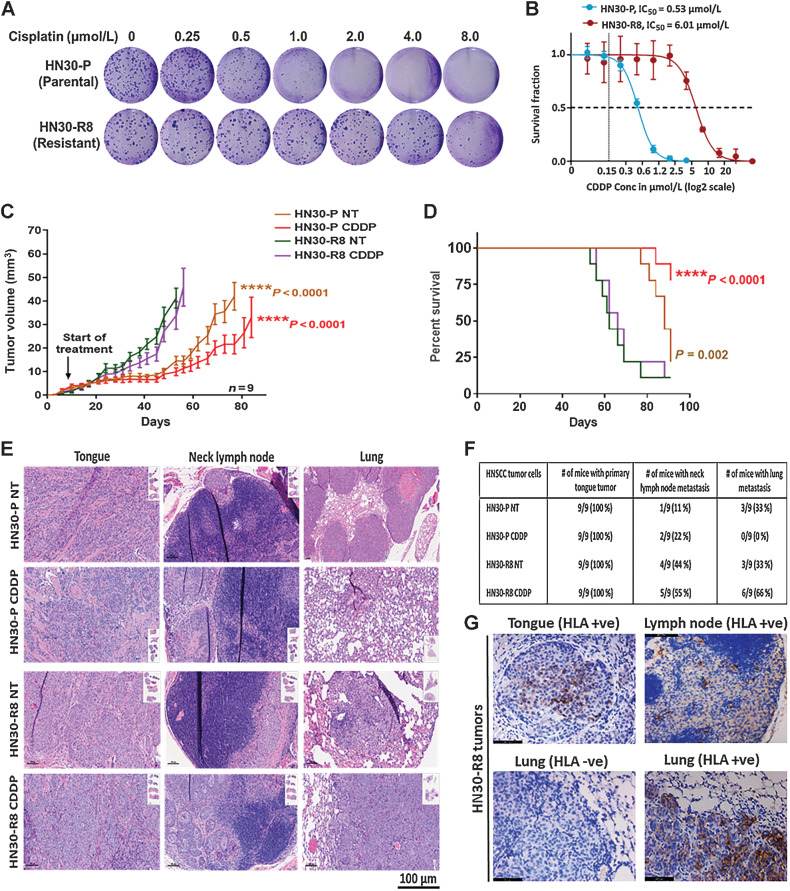
Figure 1.
Acquired cisplatin resistance enhances tumor formation and DM in vivo in an orthotopic mouse model of oral tongue cancer. HN30-P and HN30-R8 cells were plated on 6-well dishes, treated with various concentrations of CDDP in vitro, and subjected to clonogenic survival assays as indicated. A and B, Representative images of clonogenic survival and curves indicating decreased cisplatin sensitivity in CDDP-resistant HN30-R8 cells. HN30-P and its CDDP-resistant derivative, HN30-R8, were orthotopically injected into the tongues of male athymic nu/nu mice and treated intravenously via tail injection with 4 mg/kg of CDDP for 4 weeks. Tumor growth was routinely monitored with a standard caliper and is reported as tumor volume means ± SEM. C, Tumor growth curves calculated after 4 weeks of injection. Statistical analysis was performed by a one-way ANOVA test. ****, P < 0.0001 CDDP resistant versus sensitive. D, Kaplan–Meier survival curve for OS of mice injected with HN30 parental or CDDP-resistant cells with and without CDDP treatment. E, H&E representative images of the primary tongue, lymph, and lung metastatic tumors. F, Percentage of tumor incidence in tongue, neck lymph nodes, and lungs in mice treated with CDDP. G, Representative images of positive human HLA immunostaining confirming the human cell origin of the metastases in the lymph nodes and lungs. The HLA antibody staining is shown in brown and DAPI counterstain is shown in blue.