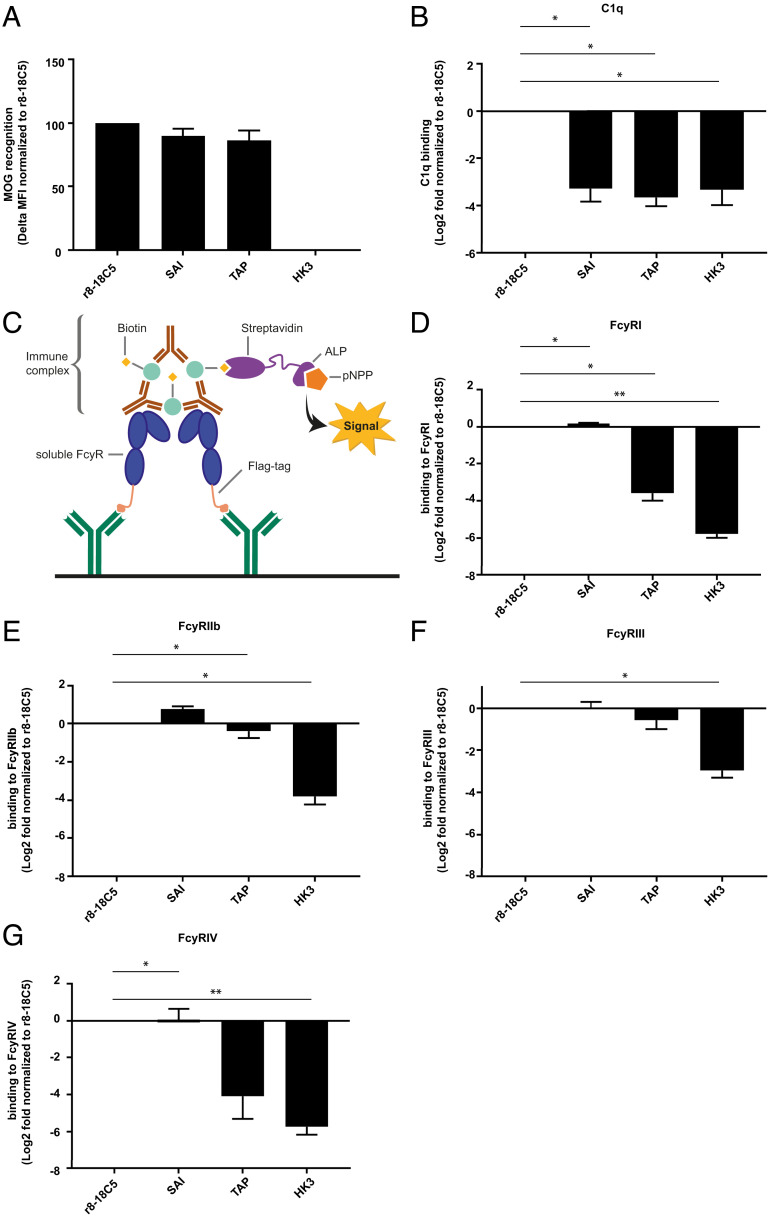
Fig. 1.
Antigen recognition, C1q- and FcγR-binding of mutated MOG-specific mAbs. The MOG antibody r8-18C5 and the Fc mutant antibodies SAI and TAP show comparable binding to MOG in a cell-based flow cytometry assay (A). IgG binding to MOG was normalized to binding of the r8-18C5 and the assay was repeated two times for each antibody (delta MFI value). (B) C1q binding was analyzed with an ELISA. Specifically, ED-hMOG was coated and indicated Ab variants were added. Subsequently, C1q was added and its binding was quantified using anti-C1q Ab. Binding of the mutant Abs was normalized to the binding of the antibody r8-18C5 and the assay was repeated three times for each antibody. FcγRI, FcyRIIb, FcyRIII, and FcγRIV binding was investigated by ELISA (C–G). Anti-FLAG antibodies were coated on an ELISA plate and used to capture soluble flag-tagged FcγR (FcγRI, FcyRIIb, FcyRIII, FcγRIV) (C). Binding of antibody-MOG IgG immune complexes to the added soluble FcγRs was quantified (D-G). Binding of the mutant MOG-Abs SAI and TAP is normalized to the binding of the MOG antibody r8-18C5. The assay was repeated three times for each antibody. Data are shown as mean with SEM. Each mutant mAbs’ affinity to C1q and FcyRs was presented as log2 fold change compared to r8-18C5 measured on the same plate and was analyzed using 2-tailed one-sample t test (parametric Welch’s t test) with Benjamini–Hochberg multiple comparison correction (n = 3).