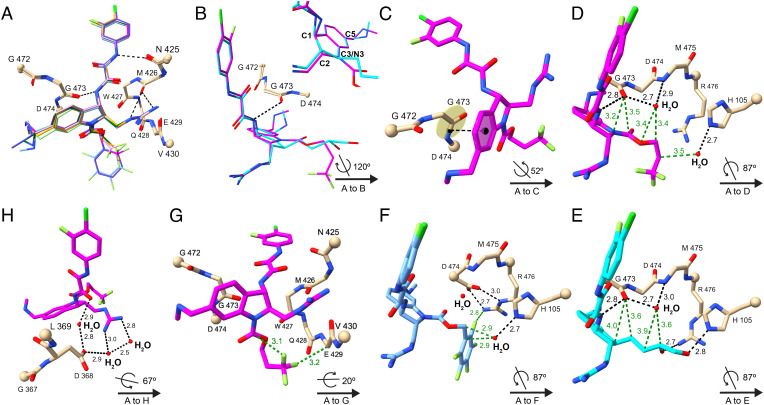
Fig. 5.
Crystal structures of CD4mcs in complex with HIV-1C1086 gp120 coree. (A) Comparison among crystal structures of indoline CD4mcs after superimposition of the gp120 cores: CJF-III-214 (17), light green; CJF-III-289 (18), dark green; CJF-III-288 (19), orange; CJF-III-192 (26), purple; CJF-IV-047 (28), pink; DY-III-065 (29), magenta; and CJF-IV-046 (30), light blue. Carbon atoms of gp120 are in beige; other atoms are colored as in Fig. 4. The Cα atoms of terminal residues in gp120 segments are represented in balls. A key is given at lower right in each of panels (B)–(H) for the orientation of its structure relative to that of (A). (B) Comparison between indoline CD4mc DY-III-065 (29) and indane CD4mc CJF-III-049-S (2) after superimposition of the gp120 cores. The carbon atoms of DY-III-065 and CJF-III-049-S are in magenta and cyan, respectively. The inset image compares DY-III-065 and CJF-III-049-S after superimposition of their aromatic six-membered rings. (C) π–π interactions between the aromatic six-membered ring of DY-III-065 (29) and the G473-D474 peptide unit of gp120. The black dashed line connects the centroid of the aromatic six-membered ring of DY-III-065 and center of the G473-D474 peptide bond. (D–F) Hydrogen bonds and nonbonded contacts that DY-III-065 (D), CJF-III-049-S (E), or CJF-IV-046 (F) form with gp120 and surrounding water molecules. Hydrogen bonds and nonbonded contacts are shown as black and green dashed lines, respectively. (G) Fluorine atoms of DY-III-065 (29) form favored nonbonded contacts with the Cα of E429 in gp120 and with its own carbamate carbon. (H) A water network at the protein–ligand interface in the structure of the DY-III-065 (29) complex associates the strictly conserved gp120 D368 carboxylate with the guanidinium group from C2 of the CD4mc.