Abstract
Dynamic chromatin accessibility regulates stem cell fate determination and tissue homeostasis via controlling gene expression. As a histone‐modifying enzyme that predominantly mediates methylation of lysine 27 in histone H3 (H3K27me1/2/3), Polycomb repressive complex 2 (PRC2) plays the canonical role in targeting developmental regulators during stem cell differentiation and transformation. Embryonic ectoderm development (EED), the core scaffold subunit of PRC2 and as an H3K27me3‐recognizing protein, has been broadly implicated with PRC2 stabilization and allosterically stimulated PRC2. Accumulating evidences from experimental data indicate that EED‐associating epigenetic modifications are indispensable for stem cell maintenance and differentiation into specific cell lineages. In this review, we discuss the most updated advances to summarize the structural architecture of EED and its contributions and underlying mechanisms to mediating lineage differentiation of different stem cells during epigenetic modification to expand our understanding of PRC2.
Polycomb repressive complex 2 (PRC2) is composed of four core subunits – embryonic ectoderm development (EED), enhancer of zeste homologue 2, suppressor of Zeste 12, and RB‐binding protein 4/7. PRC2 catalyses methylation of H3K27, an enzymatic activity necessary for PRC2‐mediated epigenetic gene silencing during stem cell differentiation and transformation. PRC2 absent EED is incapable of recognizing binding sites to promote deposition of H3K27me3, results in a significant upregulation of genes that are normally associated with H3K27me marks.

1. INTRODUCTION
Organ morphogenesis and functional maturation involve a series of lineage choice and cell‐fate decisions, which require tight spatial and temporal regulation of gene expression, activating the transcription of lineage‐specific transcriptional programmes and simultaneously repressing lineage‐inappropriate gene expression. The nucleosome represents the smallest repeated structural unit of eukaryotic chromatin, consisting of DNA wrapping around histones, 1 , 2 while transcription factor (TF) specificity and chromatin accessibility are affected by epigenetic mechanisms. 3
Polycomb group (PcG) proteins are key epigenetic regulators that mediate heritable transcriptional silencing by modifying chromatin states and participating in the establishment and maintenance of cell fates during multicellular development. 4 , 5 , 6 , 7 , 8 They are identified originally in Drosophila as repressors of Hox genes and are gradually found in other species, such as human, mouse, Xenopus, and so on. 9 , 10 PcG proteins have garnered attention for their ability to modulate mammal stem cell differentiation. Generally, there are two types of polycomb proteins in mammals, polycomb repressive complexes 1 and 2 (PRC1 and PRC2), catalysing the ubiquitylation of histone 2A at lysine‐119 (H2AK119ub) and the trimethylation of histone 3 at lysine‐27 (H3K27me3), respectively. 11 PRC2 is composed of four core subunits, enhancer of zeste homologue 1/2 (EZH1/2), embryonic ectoderm development (EED), suppressor of Zeste 12 (SUZ12) and RB‐binding protein 4 or 7 (RBBP4/RBAP48 or RBBP7/RBAP46). 7 EZH1/2 are homologous analogs, and EZH1 can partially compensate for EZH2 function in some developmental cells. 12 , 13 , 14 , 15 The SET‐domain containing protein EZH2 endows the Polycomb PRC2 complex with histone lysine methyltransferase activity, but its activity requires the participation of the other three subunits of the core complex. 16 , 17 EZH2 displays an autoinhibited state and exhibits little histone methyltransferase activity when on its own. 18 In mammals, The WD‐repeat‐containing protein EED is present as four distinct isoforms, which are believed to be mediated by utilizing four in‐frame start codons of a single EED mRNA transcript. 19 One common critical function of the EED isoforms is stabilizing EZH2 in the PRC2 complex. In addition, the PRC2 complex binds to H3K27me3 through the aromatic cage of EED, which has specific recognition of defined (repressive) trimethylated‐lysine residues. 20 , 21 SUZ12 stabilizes PRC2 by interacting with EZH2 via its VRN2‐EMF2‐FIS2‐Suz12 box (VEFS) domain. 22 , 23 Furthermore, SUZ12 is responsible for the methylation of lysine 9 of histone 3. 24 As the fourth core subunit of PRC2, the histone‐binding protein RbAp46/48 is essential for PRC2 binding with histone tails. 25 Recruitment of the PRC2 complex to chromatin is mediated by interaction with non‐core subunits such as Zinc finger protein AE binding protein 2 (AEBP2), Polycomb‐like homologues (PCLs), PRC2‐associated LCOR isoform 1 or 2 (PALI1/2), Polycomb repressive complex 2‐associated protein (EPOP), and Jumonji and AT‐rich interaction domain containing 2 (JARID2). 7 , 26 , 27 , 28 , 29 Non‐core subunits compete to bind to the N‐terminal region of SUZ12, which defines the PRC2.1 and PRC2.2 subcomplexes. PRC2.1 contains one of the three PCLs with either EPOP or PALI1/2, while PRC2.2 contains AEBP2 and JARID2 (Figures 1 and 2A). 28 , 29 , 30 , 31
FIGURE 1.

Subunits composition, chromatin association and protein domain structure of Polycomb repressive complex 2 (PRC2). (A) Schematic drawing of PRC2.1 and PRC2.2 core subunits and accessory subunits. Single‐headed arrows represent the deposition of histone marks, and double‐headed arrows depict chromatin binding. (B) Domain structure of PRC2 core subunits. Linker regions are omitted.
FIGURE 2.

(A) Crystal structure of the Polycomb repressive complex 2 (PRC2) in the basal state. Components of structures are colour‐coded. Structure figure is rendered in PyMOL. PDB ID:5KJI.155. (B) Structure showing embryonic ectoderm development (EED) in complex with the N terminus of EZH2. Structure figure is rendered in PyMOL. PDB ID:2QXV.44.
A vast number of studies report that the core subunits of the PRC2 complex show spatiotemporal specific expression patterns, indicating that they may have distinct functions. 32 , 33 , 34 , 35 EZH2 is highly expressed in proliferating cells, while EZH1 is at high levels in the differentiated tissues. 15 , 36 EED is generally regarded as a gene silencing regulator that maintains pluripotency of embryonic stem cells (ESCs) and cell proliferation. Furthermore, increasing evidence suggests that EED contributes to stem cell maintenance and lineage specification in ontogenesis. 34 , 37 , 38 , 39 , 40 , 41
This review provides a comprehensive overview of EED in regulating stem cell fate and the underlying regulatory mechanisms during various organ morphogenesis, refining the framework for understanding the functions and mechanisms of PRC2‐mediated regulation of gene expression.
2. THE STRUCTURAL BASIS OF EED
As a WD‐40 repeat family protein, EED contains seven WD40‐repeat motifs at its C terminus preceded by an extended N‐terminal segment. 20 A WD40 repeat also called a WD or β‐transducin repeat is a short, ∼40‐residue motif. 42 , 43 The WD40 repeats of EED were multimerized to fold into a canonical seven‐blade β‐propeller structure. The seven blades are radially arranged around a central axis to form a peptide‐binding pocket in the centre of the β‐propeller structure. 20 EED interacts with the N‐terminal domain of EZH2 through its larger bottom surface of WD40 repeat motifs, which in turn modulates EZH2's histone methyltransferase activity. 44 An aromatic cage is a common trait for most methyllysine‐binding motifs, 45 and EED recognizes H3K27me3 through the aromatic cage located on the top surface of its WD40 repeat domain. 46 , 47 Through the trimethyllysine, the histone peptide binds to EED and is recognized by the aromatic cage to form EED–H3K27me3 peptide complex structure. 20 Thus, EED acts not only as a critical molecule to compose the PRC2 complex but also as an ‘epigenetic exchange factor’ to modulate methylation on histones.
3. EED MODULATES ALLOSTERIC ACTIVATION AND RECRUITMENT OF PRC2
EZH2 adopts an autoinhibitory conformation through crystal structures of the inactive isolated catalytic domain, suggesting that structural rearrangement of EZH2 is likely required for PRC2 activation. 18 , 47 Moreover, a range of crystallographic structures of EED in complex with EZH2, trimethylated histone and nonhistone peptides highlight the pivotal role of EED in mediating EZH2 binding and triggering PRC2 an allosteric activation of catalysis. 20 , 44 , 48 , 49 After PRC2 complex assembly, the activation loop from the N‐terminal portion of EZH2 is moved by EED to the neighbouring catalytic SET domain (Figure 2B). 45 The EED‐binding domain (EBD) of EZH2 occupies the bottom face of the EED WD40 domain, and then three β strands are added to EED to form the β‐addition motif (BAM) and maintain EED in a stable position to allow H3K27me3 binding to the top WD40‐repeat domain of EED (Figure 3A). 47 , 50 The SET activation loop (SAL) is formed after the BAM migrates away from the EED surface to the back of the SET domain of the catalytic moiety. Then the H3K27me3 peptide is sandwiched between EED and an exposed EZH2 motif, referred to as the stimulation‐responsive motif (SRM), and immediately follows the SAL of EZH2. SRM of the sandwich‐like assembly interacts extensively with two others and transforms itself into a fully ordered α‐helix‐loop structure. 47 Finally, the interaction between SAL and the newly formed SRM helix of EZH2 stabilizes the active conformation of the EZH2 SET, resulting in enhanced histone methyltransferase activity of PRC2. In addition, EED along with the SAL and SET regions of EZH2 get glued together by the N‐terminal loop region of SUZ12 (VEFS). Additionally, the SANT1‐binding domain (SBD) of EZH2 contacts with the DNA in the H3K27me3‐marked nucleosome after binding by EED. 47 , 51 Through a highly complex series of EED, EZH2, and H3K27me3 interactions, EED allosterically regulates PRC2 histone methyltransferase activity and PRC2 on‐chromatin spreading. 20 , 52 , 53
FIGURE 3.

(A) Structure showing embryonic ectoderm development (EED) in complex with H3K27me3. Structure figure is rendered in PyMOL. PDB ID:3JZG.48. (B) Structure showing EED in complex with JARID2. Structure figure is rendered in PyMOL. PDB ID:4X3E.49. (C) Crystal structure of EED in complex with PALI1‐K1219me3 peptide. Structure figure is rendered in PyMOL. PDB ID:6V3Y65. (D) Crystal structure of EED in complex with PALI1‐K1241me3 peptide. Structure figure is rendered in PyMOL. PDB ID:6V3X.65.
The PRC2 crystal structures provide fundamental insights into the interaction with each subunit, substrate recognition, and allosteric activation of its enzymatic activity. Moreover, PRC2 is more active on di‐nucleosomes and higher‐order chromatin structures than on mononucleosomes or histone tails, 50 , 54 , 55 indicating the crucial role of interaction with the chromatin in PRC2 activation. A response to allosteric stimulation of PRC2 results in it catalysing H3K27me3 on neighbouring nucleosomes, causing the formation of broad H3K27me3 domains. 56 Recently, a cryo‐EM structure of PRC2 in di‐nucleosomes revealed that EED engages with one H3K27me3‐modified nucleosome to allow the H3K27me3 thread into the aromatic cage of EED and locates the SET domain present in EZH2 to methylate an unmodified H3 tail on the other adjacent nucleosome. 50 EED and EZH2 consist of the dominant interface to interaction with di‐nucleosome, while SUZ12, RbAp46/48, AEBP2 and JARID2 could also potentially assist nucleosome interaction. 57 These structures provide additional mechanistic explanations for the PRC2 complex interacting predominantly with the nucleosome, which is quite different from most other chromatin modifiers interacting with DNA and the conserved histone acidic patch surface.
PRC2 recruitment is modulated by many different factors, including the interaction of PRC2 subunits with DNA and histones, PRC1‐mediated H2AK119ub1, RNA and other histone modifications. 58 , 59 PRC2 recruitment involves two main functional axes, one is MTF2‐PRC2.1 binding to DNA, and the other is JARID2 binding to the marker H2AK119ub1, from PRC1. 60 They are both reinforced by H3K27me3‐EED‐positive feedback. JARID2 incorporating the complex confers PRC2 partial function of the initiate silencing. 49 Through binding to the aromatic cage of EED and remodelling the SRM domain of EZH2, JARID2 is trimethylated at lysine 116 mimics (JARID2K116me3) (Figure 3B) which has an allosteric stimulatory effect on PRC2 histone methyltransferase activity. 61 It is noteworthy that JARID2‐K116me3 promotes the allosteric activation of PRC2 but does not participate in H3K27me3, which is invoked as one of a mechanism for PRC2 deposition to unmodified nucleosomes. 62 When H2AK119ub1 enriches at CpG islands (CGIs), EED‐mediated JARID2 cooperation with H2AK119ub1 could contribute to the CGI preference of PRC2. 63 , 64 More recently, the trimethylated state of PALI1 has also been shown can allosterically activate the PRC2 complex when bound to EED through a similar mechanism to that proposed for H3K27me3 (Figure 3C,D). 65
4. THE INTERACTION OF EED AND H3K27ME3
Mammalian heterochromatin contains great repressive chromatin domains, including H3K9me2/3‐modified constitutive heterochromatin and H3K27me2/3‐decorated facultative heterochromatin. The two categories of histone modifications are catalysed by Suv39h1/2 and PRC2, respectively. 66 , 67 PRC2 deposits the repressive histone mark H3K27me3 through an aromatic cage of EED and establishes the direct interaction with its target genes. 20 Furthermore, PRC2 binding or catalytic activity is also affected by histone modifications in the chromatin region, one of which is H3K27me3. 68 Aranda et al. report that EED has high‐affinity binding for histone methylations correlated with transcriptional repression. 69 EED can bind H3K27me3 when H3K27me3 is present on two adjacent nucleosomes, and the presence of H3K27me3 stimulates PRC2 enzyme activity, thus generating a positive feedback loop. Mutations of the EED aromatic cage or EED absence will disrupt this interaction and lead to a global loss of H3K27me3. 47 , 52 , 70 , 71 Other repressive chromatin marks that can be recognized by EED, such as H3K9me3, H4K20me3 and H1K26me3, indicate that recognition of repressive histone marks via EED could serve as a mechanism of PRC2 recruitment to silenced loci. 20 , 48 , 72
5. EED FUNCTIONS IN ONTOGENESIS
For pluripotent stem cells to expand and differentiate into one or more specialized and committed cell types in the body, critical cell fate decisions and lineage commitment must be made during growth and development. As differentiating stem cells undergo the cascade of lineage decisions on branching points through epigenetic‐threshold modulation, specific genes must be switched on and genes associated with alternative lineages need to be repressed in a dynamic and timely manner. Next, we will dissect the dynamic roles of EED during ontogenesis to further insight into the PRC2 and its role in the specificity and diversity of lineage specification (Figure 4).
FIGURE 4.
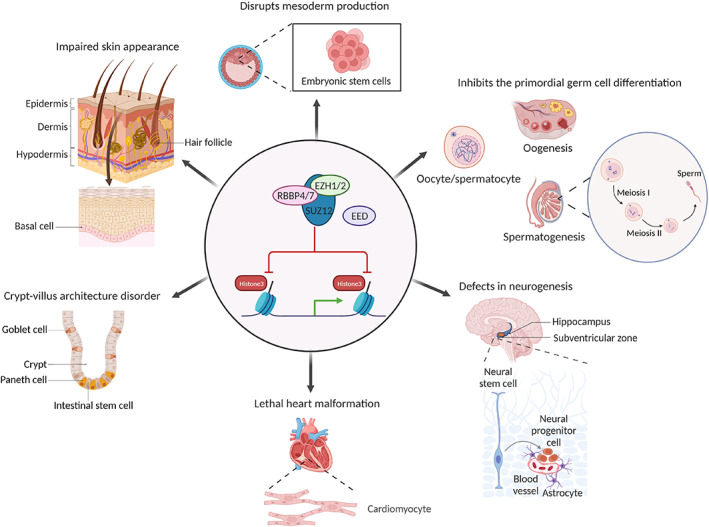
Embryonic ectoderm development (EED) protein is reside in various organ morphogenesis, including embryonic development, spermatogenesis, oogenesiscy, neurogenesis, and so on. Polycomb repressive complex 2 absent EED results in multiple developmental abnormalities.
5.1. Embryonic development
ESCs possess the ability to differentiate into all the cell types of tissues and organs. 73 ESCs pluripotency is governed by a gene regulatory network composed of various TFs and chromatin‐modifying enzymes. 74 PRC2 complex is essential for early embryonic development and has been implicated in pluripotency as well as cell fate determination in ESCs. In human and murine ESCs, PRC2 localizes to the promoters of repressed genes, which encode TFs for specification later during development, and EED mutations cause their premature expression. 75 EED is also required for regulating normal murine embryogenesis. When mice are knocked out of EED, early embryonic lethality occurs because they fail to properly gastrulate and produce embryonic mesoderm. 76 , 77 , 78 Montgomery et al. demonstrate that EED−/− embryos result in a genome‐wide decrease in H3K27me1, H3K27me2 and H3K27me3. 19 ESCs absent EED results in inactivating histone methyltransferase and lack the H3K27me3 modification. 21 , 79 EED and H3K27 methylation are also involved in the gene regulation of undifferentiated and differentiating cells, facilitating ESCs differentiation towards a specific lineage. Through performing gene expression and chimera analyses on both low and high‐passage EED null ESCs, Chamberlain et al. identify that EED null ESCs fail to differentiate properly in vitro, but can contribute to chimeras. 80 ESCs without EED can maintain pluripotency markers and self‐renew, but fail to execute differentiation programmes promptly. 81 These results focus on studying undifferentiated ESCs. By contrast, Obier et al. perform global gene expression analysis in both undifferentiated ESCs and embryoid body formation of EED knocked out, and results demonstrated that EED is required to silence the pluripotency network during differentiation. 82 Galonska et al. find both H3K27me3 and DNA methylation absence when EED‐deficient ESCs culture in the two inhibitors (2i) conditions. 83 But the latest research shows that genome‐wide DNA methylation and H4 acetylation are increased in the EED‐deficient ESCs culture in the 2i conditions. 84 Hence, EED is critical for ESCs as it regulates both developmental control genes and a subset of canonically imprinted genes.
5.2. Spermatogenesis and oogenesis
The primordial germ cells (PGCs) are precursors of the oocyte and sperm, which transmit significant epigenetic information to the offspring. 85 , 86 , 87 High EED expression is concurrent with a high level of H3K27me3 enrichment in XX and XY PGCs during development. 88 , 89 EED is a key effector of oocyte meiosis and spermatogonia differentiation. Spermatogonia is a heterogeneous population, consisting of spermatogonia stem cells (SSCs), undifferentiated spermatogonia and differentiated spermatogonia, 90 while EED is present in the first two of the cell population. 91 There are also EED and H3K27me3 enriched throughout the gene body in spermatocytes, covering both introns and exons. 92 Differential H3K27me3 enrichment at retained nucleosomes in sperm indicates that heritable epigenetic information could affect paternal offspring. 93 , 94 EED prevents precocious differentiation of XY and XX PGCs through responding to sex‐specific developmental signals, and EED/H3K27me3 deficient from PGCs chromatin involves itself in a synergistic pathway with H2AK119ub1 and DNA methylation to regulate the PGCs response to the niche during sex determination in the embryonic testis and ovary. 92 By ablation of EED in the germ cells specifically, spermatocytes decrease dramatically, and eventually, no spermatids or post‐pachytene spermatocytes remain, indicating that EED is required for meiotic progression. 91 Besides, both SSCs and undifferentiated spermatogonia markers Lin28a, Ngn3 and Nanos3 were significantly decreased in EED mutants. Loss of EED results in SSCs deficiency and abnormal meiotic chromosome dynamics, ultimately leading to male infertility. Stringer et al. highlight that sporadic sub‐fertility of lacking EED in the paternal germline produces sub‐fertile male offspring, involving de‐repression of both LINE elements and retrotransposed pseudogenes. 94 Impressively, Oocyte‐specific deletion of EED results in H3K27me3 absent and a significant overgrowth of offspring, which involves both adiposity and bone mineral density increase. 95 Recent research has shown that loss of EED in somatic and germ cells leads to abnormal ovaries in adult mutant females, but the mutants are fertile. 96 Overall, epigenetic inheritance altered by EED is important to spermatogenesis and oogenesis, especially regulating repetitive sequences in the paternal germline.
5.3. Neurogenesis
During the development of the nervous system, the PRC2 complex plays a vital role in maintaining the self‐renewal and proliferation capacities of neural stem/progenitor cells (NSPCs). 97 Embryonic neurogenesis is initiated in the neuroepithelial cells of the ventricular zone (VZ), and the subventricular zone (SVZ) differentiates into radial glial cells (RGCs). RGCs can either directly produce neurons or generate neuronal intermediate progenitor cells, which differentiate into neurons, astrocytes and oligodendrocytes. While in the adult brain, NSPs are in the SVZ and the subgranular zone of the hippocampal dentate gyrus (DG). 98 EED is indispensable for spinal cord development and NSPCs proliferation in the SVZ region. Partial mutations in genes encoding the EZH2 or EED subunits lead to Weaver syndrome, 99 characterized by variable intellectual disability and distinctive facial features. EED is highly expressed in the brain and involved in the differentiation and maturation process of the central nervous system cells. Downregulation of EED in the spinal cord and neural tube inevitably leads to spina bifida and neural tube defects. 100 An early study has been done to characterize EED as a key regulator of neurogenesis using EED‐deficient mice. 101 Conditional knocked out (cKO) of EED in neural progenitors of the neocortex leads to a prolonged neurogenic phase and deferring astrocytes differentiation. 97 Sun et al. report that EED promotes TF Gata6 expression and reduces p21 protein level when EED is deleted in neural stem cells of the SVZ, indicating the importance of EED to NSPCs proliferation and neurogenesis in the SVZ. 102 Besides, the proper formation of DG is also required for EED. In the absence of EED in the NSPCs, cyclin‐dependent kinase inhibitor 2 A (Cdkn2a) expression is increased and critical gene SRY‐box transcription factor 11 (Sox11) for neural differentiation is repressed, which ultimately leads to shorter and smaller dentate gyrus formation, indicating that EED primarily acts as an activator for maintaining proliferation and differentiation. 39 EED is essential for oligodendrocyte (OL) remyelination, while it is dispensable for myelin maintenance. EED deletion in OL progenitors results in H3K27me3 absent and OL lineage abnormalities. 40 Yaghmaeian and colleagues also find H3K27me3 disruption in the developing mouse hypothalamus when EED is cKO, which triggers reduced cell proliferation, ectopic expression of posteriorly expressed regulators and increased expression of cell‐cycle regulators. 103 In addition to NSPCs and oligodendrocytes, recently, microglial EED has been demonstrated to be essential for synaptic pruning during normal postnatal brain development. 104 Furthermore, deletion of EED in the forebrain leads to the upper‐layer neuron numbers being significantly decreased and abnormal cortical architecture. Genomic and transcriptomic network analyses indicate that abnormal acetylation of H3K27 (H3K27ac) accumulation is associated with the decrease of H3K27me3 and the recruitment of RNA‐Pol2. 41 Consequently, EED has multiple functions in neurogenesis, and it plays dynamic roles in NSPCs maintenance and the stage of gliogenesis.
5.4. Cardiogenesis
The development and function of the cardiovascular system are vulnerable to epigenetic insults, one of which is epigenetic repressors PRC2. 105 , 106 , 107 In the murine hearts, alterations of chromatin landscape have repeatedly been linked to both cardiomyopathy and structural heart disease in postmitotic cardiomyocytes. 37 , 106 , 108 , 109 Moreover, the inactivation of EED in murine foetal cardiomyocytes leads to cardiac fibrosis and significant systolic impairment. During early heart development, loss of H3K27me3 is complete upon cardiac‐specific inactivation of EED, resulting in lethal heart malformations. 105 The previous study has shown that EED interacts with significant amounts of proteins in the Endothelin‐1‐induced cardiomyocyte proteome. 110 EED also interacts with phospholipase neutral sphingomyelinase 2 (N‐SMase2) through coupling TNF‐R1 to N‐SMase2, thus mediating heart failure and atherosclerosis. 111 Using Tie2Cre to inactivate the floxed murine EED results in absence of blood‐perfused vasculature via exhausting the haematopoietic stem cells pool. 112 Ai et al. highlight that in EED cKO mice, significant loss of H3K27me3 can be observed, but it does not directly regulate the upregulation genes, which include upregulated skeletal muscle genes. In contrast, abnormal H3K27ac accumulation is associated with these upregulated genes, indicating that EED complexes with and stimulates HDAC deacetylase activity to silence the slow‐twitch myofiber gene programme to orchestrate heart maturation. 37 Furthermore, with absent EED in postmitotic cardiomyocytes of adult mouse hearts, Li et al. find a long lifetime of cardiomyocytes accompanied by a short histone half‐life. 113 Mechanistically, EED ablation is involved in histone flux and nucleosome remodelling through BRG1, which results in the inhibition of nucleosomal histones exchange and histone turnover, eventually leading to H3K27ac accumulation. Besides, EED‐knockdown in human pluripotent stem cells significantly enhanced cardiac differentiation with increasing expression of multiple cardiac genes, such as myocyte enhancer factor 2C (MEF2C), myosin heavy chain 6/7 (MYH6/7), cardiogenic TFs NKX2.5 (NKX2‐5), and so on. 114
5.5. Intestinal morphogenesis
Intestinal stem cells (ISCs), located at the bottom of the crypts, are a cell population self‐renew extensively and differentiates into all cell types within crypts and villi. 34 , 115 , 116 , 117 In the developmental and adult intestine, PRC2‐mediated H3K27me3 deposition is required for transitions from ISCs to transit‐amplifying progenitors and post‐mitotic villus cells. 34 , 116 , 118 It is estimated that about 2000 genes are marked by H3K27me3 in both crypt and villus cells. 119 By cKO EED in the intestine, Chiacchiera et al. report that crypt‐villus architecture disorders attributed to EED affect cell cycle progression and differentiation of transient amplifying cells at the crypt bottom instead of ISCs maintenance. 116 Conversely, Koppens and colleagues find EED deletion in the intestine using the same animal model results in uncommitted crypt cells in an aberrant differentiation and reduced cell proliferation. 34 More crucially, the loss of ISCs and inhibition of Wnt signalling are also highlighted in this paper. The difference should be expected due to a compromised response to the long‐time βNF administration, which might affect the ISCs compartment. But the two studies coincide in the most striking result: Loss of EED causes inactivation of PRC2, and the cell cycle is arrested in crypts, which can be attributed to deleting and de‐repression of Cdkn2a. Surprisingly, genes essential for intestinal development are silenced by H3K27me3 in the adult intestinal epithelium but reactivated without PRC2 action, which is associated with functional interactions of H3K27me3 with H3K4me3. 120 A novel observation reveals that the absence of EZH2 and H3K27me3 in EED‐null villi cells results in stunted and dysmorphic villi. 121 By performing absenting EED in distinct cell compartments of the intestinal epithelium, the further investigation reveals that H3K27me3 loss occurs as a result of replicational dilution, which occurs proportional to the frequency of cell division.
5.6. Skin and hair follicle morphogenesis
The skin is the first barrier that protects mammals against external insults and dehydration. Epidermal lineages derive from a single layer of multipotent skin progenitors named basal cells, which attach to underlying basement membranes separating the epidermis from the dermis. 122 , 123 The basal cells generate the hair follicles, the sebaceous glands, the interfollicular epidermis, and the Merkel cells. 124 , 125 Among these, the hair follicles contain stem cells of the dermal and epidermal lineages. 126 EED is expressed mainly in basal epidermal cells while downregulated upon differentiation during developing skin. 127 Besides, EED and H3K27me3 are downregulated, whereas H3K27me3 demethylase UTX (lysine demethylase 6A, KDM6A) and JMJD3 (lysine demethylase 6B, KDM6B) are enriched during mice skin repair, indicating that losing polycomb‐mediated silencing may be involved in the induction of repair genes. 128 EED‐specific deletion in the skin epithelium contributes to premature epidermal barrier development, ectopic Merkel cell formation, and postnatal hair follicle developmental hurdle. 129 Further study indicates all hair follicle types but not just primary hair follicles, have the potential to induce Merkel cells when lacking EED in the skin epidermis. 130 EED absence leads to the de‐repression of Sox2, Atoh1 and Isl1, the master TFs required for normal Merkel cell differentiation. Interestingly, deletion of EED decreases the proliferation of hair follicle progenitor cells rather than basal cells, indicating there may be a mechanism compensating for the loss of EED function in the epidermal progenitor cells. A recent study has provided an explanation for this phenomenon. Cohen et al. ablate both PRC1 core (Ring1a and Ring1b) and PRC2 core (EED) subunits in embryonic epidermal stem cells (EpSCs) using Krt14‐Cre mice. Neither H2AK119ub nor H3K27me3 histone can be detected in the EED; Ring1a/b 3KO epidermis. 131 The mutant mice died shortly after birth with a visibly impaired skin appearance. Loss of both PRC1/2 complexes in EpSCs leads to the key epidermal lineage TFs such as P63 and SATB1 being significantly down‐regulated in EpSCs, indicating functional redundancy between PRC1 and PRC2 in the maintenance of epidermal stem cell identity. Moreover, EED absences in adult hair follicle stem cells (HFSCs) cannot induce HFSCs activation or fate switch. 132 In contrast, loss of EED function in adult epidermal stem cells results in epidermal pigmentation. 133 These studies highlight the functional complexity of EED in different cell lineages of the developing and adult epidermis.
5.7. Haematopoiesis
The bone marrow (BM) contains haematopoietic and mesenchymal stem cells. 134 , 135 Haematopoietic stem cells (HSCs) of neonatal BM self‐renew rapidly to expand the HSC pool during the growing stage. 136 , 137 , 138 However, adult BM HSCs remain quiescent and demonstrate a capacity to regenerate after injury. 139 , 140 EED has high expression in neonatal and adult HSCs. 38 , 138 Haematopoiesis defects are observed in the BM and thymus of the EED mutant animals. 141 , 142 VavCre‐mediated EED excision appeared neonatal pale and hypocellular, leading pups dead within 5–12 days after birth. 138 EED deletion in embryos perturbs the BM HSCs differentiation into restricted lineage progenitor cells. 112 At the same time, loss of EED in adult BM HSCs enhances gene expression associated with proliferation and differentiation, which results in the exhaustion of HSCs. 138 Ueda et al. generate EED mutants by knock‐in I363M to impair the EED structural integrity and find mutated‐homozygotes development arrest at E14.5. 143 Further analysis shows that dramatically reduced H3K27me3 rather than H3K27me1/me2 or H3K4me3 occurs in EED homozygous mutants, indicating the structural integrity of EED is essential for the H3K27me3 mark. Interestingly, EED heterozygous mutants can survive the whole lifespan but undergo age‐dependent expansion and hyperproliferation of BM haematopoietic progenitors, increasing susceptibility to hematologic malignancies. 141 , 143 , 144 Therefore, EED complete and partial loss‐of‐function appear to have different effects on BM haematopoiesis.
5.8. Osteogenesis
Bone formation involves two major distinct mechanisms: intramembranous and endochondral ossification. 145 , 146 Cartilage is gradually replaced by bone via endochondral ossification to form the most mammalian skeleton. 147 Besides, mesenchymal cells that originate from the neural crest are responsible for the development of the craniofacial skeleton. 148 De novo germline mutations in the human EED gene lead to Weaver syndrome, a disease characterized by skeletal defects, advanced bone age and overgrowth. 149 , 150 , 151 , 152 EED interacts with Bmi1 in genetic, biochemical and molecular to mainly regulate an overlapping set of Hox genes, thus avoiding developmental abnormalities of the vertebra. 153 Absent EED in chondrocytes causes efficient elimination of H3K27me3, severely deformed thoracic spine, and shortening the long bones. In EED cKO mice, hypoxia‐inducible transcription factor 1α (Hif1a) down‐regulation induces cell death in the central area of epiphyseal growth plates. In contrast, overactivation of Wnt signalling leads to premature hypertrophic differentiation and premature growth plate closure. 35 Moreover, H3K27me3 and H3K27me3 demethylase UTX enrichment in cartilage correlates are linked to osteoarthritis. The latest study shows that EED‐blocked expression is associated with UTX loss‐induced H3K27 hypomethylation, which showed few gonarthrotic symptoms in collagen‐induced arthritis. 154 Collectively, EED regulates early mesenchymal lineage to differentiate towards the osteogenic lineages, thereby having a great influence on bone formation, but further investigation is need to elucidate the specific mechanism.
6. CONCLUSIONS
In this study, we comprehensively and systematically reviewed the research advances on EED/PRC2 function regulating ontogenesis (Table 1). PRC2 complex is the master epigenetic regulator of developmental and cell identity genes. As a core scaffold subunit of PRC2, EED plays separate and specific roles in different lineages and various time points. In this review, detailed biochemical and structural characterization of EED has expanded current views regarding how EED cooperates with other subunits forming PRC2 chromatin domains to catalyse histone modifications and engage with nucleosomes. We also have highlighted the numerous phenotypes of EED mutants during the development of different tissues and organs and summarized the general mechanisms of the role of EED in gene regulation in distinct lineage cells. Although much of our understanding of the EED has come from studies on various stem cells and specific tissue development, the mechanisms that guide EED in maintaining tissue homeostasis of adult organisms are likely to vary considerably. There is undoubtedly considerable scope for further research to better understand the function and regulation of EED in adult stem cells. Another optimization goal for future studies would be understanding the spatiotemporal‐specific changes in cellular EED levels, this will include improving our knowledge of the distinct functions of PRC2 core subunits. The variety of functions played by EED further expands the complex yet fascinating mechanisms and principles of PRC2‐mediated gene regulation during embryogenesis and organogenesis. In summary, insights provided in the present review will endow researchers concise understanding of the role of EED‐mediated epigenetic regulations in development. With the continuous improvement of molecular tools and sequencing technologies, the opportunity for future research into the dynamic role of EED, as well as its contribution to the development of organs and the progression of numerous diseases remains wide open.
TABLE 1.
Functional regulation of embryonic ectoderm development (EED) in ontogenesis.
| Ontogenesis | Regulated function | References |
|---|---|---|
| Embryonic development | Target genes are de‐repressed | Faust et al. 76 |
| Genome‐wide decrease in H3K27me1, H3K27me2 and H3K27me3 | Montgomery et al. 19 | |
| Decrease in Ezh2 protein levels. Disrupted axial patterning | Chamberlain et al. 80 | |
| Fail to properly gastrulate and to produce embryonic mesoderm | Leeb et al. 81 | |
| EED null ESCs fail to differentiate properly in vitro, but can contribute to chimeras | Obier et al. 82 | |
| EED is required to silence the pluripotency network during differentiation | van Mierlo et al. 84 | |
| Genome‐wide of DNA methylation and H4 acetylation are increased in the EED−/− 2i ESCs | ||
| Spermatogenesis and oogenesis | Inhibits the spermatogonia differentiation | |
| Impedes meiotic progression | Mu et al. 91 | |
| Synergistic with H2AK119ub1 and DNA methylation | Prokopuk et al. 93 | |
| Male infertility | Stringer et al. 94 | |
| Adult mutant females are fertile | Prokopuk et al. 96 | |
| Prevents precocious differentiation of XY and XX PGCs | Lowe et al. 92 | |
| Neurogenesis | A key regulator of the neurogenesis; prolonging neurogenic phase and deferring astrocytes differentiation | Schumacher et al. 101 |
| Spina bifida and neural tube defects | Hirabayashi et al. 97 | |
| Promotes neurosphere formation | Song et al. 100 | |
| Regulates proliferation in the telencephalon | Sun et al. 102 | |
| Shorter and smaller dentate gyrus | Yaghmaeian et al. 103 | |
| Mediates oligodendrocyte remyelination; maintains normal synaptic and cognitive functions | Liu et al. 39 | |
| Controls embryonic cortical neurogenesis | Wang et al. 40 | |
| Wang et al. 104 | ||
| Zhang et al. 41 | ||
| Cardiogenesis | Mediates heart failure and atherosclerosis | Philipp et al. 111 |
| Lethal heart malformations | He et al. 108 | |
| Participates in ET‐1 induced cardiomyocyte terminal differentiation | Shin et al. 110 | |
| Abnormal H3K27ac accumulation | Ai et al. 37 | |
| Regulates lifetime of cardiomyocytes | Li et al. 113 | |
| Enhances cardiac differentiation | Liu et al. 114 | |
| Intestinal morphogenesis | Crypt–villus architecture disorders | Chiacchiera et al. 116 |
| Uncommitted crypt cells in an aberrant differentiation and reduced cell proliferation |
Koppens et al. 34 Jadhav et al. 120 |
|
| Significant weight loss and severely degraded crypt | Jadhav et al. 121 | |
| Stunted and dysmorphic villi | ||
| Skin and hair follicle morphogenesis | Premature epidermal barrier development, ectopic Merkel cell formation, and postnatal hair follicle developmental hurdle | Dauber et al. 129 |
| Decreases the proliferation of hair follicle progenitor cells | Perdigoto et al. 130 | |
| Cannot induce HFSCs activation or fate switch; epidermal pigmentation | Cohen et al. 131 | |
| Flora et al. 132 | ||
| Li et al. 133 | ||
| Haematopoiesis | Haematopoiesis defects | Lessard et al. 141 |
| Increasing susceptibility to hematologic malignancies of EED heterozygous mutants | Majewski et al. 144 | |
| Neonatal pale and hypocellular | Xie et al. 138 | |
| Perturbs the BM HSCs differentiation into restricted lineage progenitor cells | Yu et al. 112 | |
| Ueda et al. 143 | ||
| Osteogenesis | Weaver syndrome | Cohen et al. 150 |
| Regulates vertebra development | Cooney et al. 151 | |
| Causes severely deformed thoracic spine, and shortening the long bones |
Kim et al. 153 Mirzamohammadi et al. 35 |
|
| Shows few gonarthrotic symptoms in collagen‐induced arthritis | Lian et al. 154 |
AUTHOR CONTRIBUTIONS
Liuyan Huang conceived, wrote, revised the manuscript and made the figure and table. Fanyuan Yu and Feifei Li revised the manuscript. Ling Ye, Chenglin Wang and Fanyuan Yu reviewed, revised and edited the manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
This work was supported by National Natural Science Foundation of China 81873708 (Chenglin Wang), 82201045 (Fanyuan Yu), Sichuan Province Science and Technology Program 2022JDRC0130 (Fanyuan Yu) and 2022ZYD0055 (Fanyuan Yu) and Young Elite Scientist Sponsorship Program by CAST 2022QNRC001 (Fanyuan Yu).
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest.
Huang L, Li F, Ye L, Yu F, Wang C. Epigenetic regulation of embryonic ectoderm development in stem cell differentiation and transformation during ontogenesis. Cell Prolif. 2023;56(4):e13413. doi: 10.1111/cpr.13413
Funding information Department of Science and Technology of Sichuan Province, Grant/Award Numbers: 2022JDRC0130, 2022ZYD0055; National Natural Science Foundation of China, Grant/Award Numbers: 81873708, 82201045; Young Elite Scientist Sponsorship Program by CAST, Grant/Award Number: 2022QNRC001
Contributor Information
Fanyuan Yu, Email: fanyuan_yu@outlook.com.
Chenglin Wang, Email: wxonet@163.com.
REFERENCES
- 1. Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science. 1974;184(4139):868‐871. [DOI] [PubMed] [Google Scholar]
- 2. Kornberg RD, Thomas JO. Chromatin structure; oligomers of the histones. Science. 1974;184(4139):865‐868. [DOI] [PubMed] [Google Scholar]
- 3. Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693‐705. [DOI] [PubMed] [Google Scholar]
- 4. Grimaud C, Nègre N, Cavalli G. From genetics to epigenetics: the tale of Polycomb group and trithorax group genes. Chromosome Res. 2006;14(4):363‐375. [DOI] [PubMed] [Google Scholar]
- 5. Francis NJ, Follmer NE, Simon MD, Aghia G, Butler JD. Polycomb proteins remain bound to chromatin and DNA during DNA replication in vitro. Cell. 2009;137(1):110‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyer LA, Plath K, Zeitlinger J, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441(7091):349‐353. [DOI] [PubMed] [Google Scholar]
- 7. Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aloia L, Di Stefano B, Di Croce L. Polycomb complexes in stem cells and embryonic development. Development. 2013;140(12):2525‐2534. [DOI] [PubMed] [Google Scholar]
- 9. Satijn DP, Otte AP. Polycomb group protein complexes: do different complexes regulate distinct target genes? Biochim Biophys Acta. 1999;1447(1):1‐16. [DOI] [PubMed] [Google Scholar]
- 10. Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128(4):735‐745. [DOI] [PubMed] [Google Scholar]
- 11. Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10(10):697‐708. [DOI] [PubMed] [Google Scholar]
- 12. Riising EM, Helin K. A new role for the polycomb group protein Ezh1 in promoting transcription. Mol Cell. 2012;45(2):145‐146. [DOI] [PubMed] [Google Scholar]
- 13. Abel KJ, Brody LC, Valdes JM, et al. Characterization of EZH1, a human homolog of Drosophila Enhancer of zeste near BRCA1. Genomics. 1996;37(2):161‐171. [DOI] [PubMed] [Google Scholar]
- 14. Ezhkova E, Lien W‐H, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25(5):485‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Margueron R, Li G, Sarma K, et al. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32(4):503‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antonysamy S, Condon B, Druzina Z, et al. Structural context of disease‐associated mutations and putative mechanism of autoinhibition revealed by X‐ray crystallographic analysis of the EZH2‐SET domain. PLoS One. 2013;8(12):e84147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Mierlo G, Veenstra GJC, Vermeulen M, Marks H. The complexity of PRC2 subcomplexes. Trends Cell Biol. 2019;29(8):660‐671. [DOI] [PubMed] [Google Scholar]
- 18. Bratkowski M, Yang X, Liu X. Polycomb repressive complex 2 in an autoinhibited state. J Biol Chem. 2017;292(32):13323‐13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montgomery ND, Yee D, Montgomery SA, Magnuson T. Molecular and functional mapping of EED motifs required for PRC2‐dependent histone methylation. J Mol Biol. 2007;374(5):1145‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Margueron R, Justin N, Ohno K, et al. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461(7265):762‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montgomery ND, Yee D, Chen A, et al. The murine polycomb group protein EED is required for global histone H3 lysine‐27 methylation. Curr Biol. 2005;15(10):942‐947. [DOI] [PubMed] [Google Scholar]
- 22. Rai AN, Vargas ML, Wang L, Andersen EF, Miller EL, Simon JA. Elements of the polycomb repressor SU(Z)12 needed for histone H3‐K27 methylation, the interface with E(Z), and in vivo function. Mol Cell Biol. 2013;33(24):4844‐4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brooun A, Gajiwala KS, Deng Y‐L, et al. Polycomb repressive complex 2 structure with inhibitor reveals a mechanism of activation and drug resistance. Nat Commun. 2016;7:11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de la Cruz CC, Kirmizis A, Simon MD, Isono K‐i, Koseki H, Panning B. The polycomb group protein SUZ12 regulates histone H3 lysine 9 methylation and HP1 alpha distribution. Chromosome Res. 2007;15(3):299‐314. [DOI] [PubMed] [Google Scholar]
- 25. Kuzmichev A, Margueron R, Vaquero A, et al. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci U S A. 2005;102(6):1859‐1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landeira D, Sauer S, Poot R, et al. Jarid2 is a PRC2 component in embryonic stem cells required for multi‐lineage differentiation and recruitment of PRC1 and RNA Polymerase II to developmental regulators. Nat Cell Biol. 2010;12(6):618‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li H, Liefke R, Jiang J, et al. Polycomb‐like proteins link the PRC2 complex to CpG islands. Nature. 2017;549(7671):287‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beringer M, Pisano P, Di Carlo V, et al. EPOP functionally links Elongin and Polycomb in pluripotent stem cells. Mol Cell. 2016;64(4):645‐658. [DOI] [PubMed] [Google Scholar]
- 29. Conway E, Jerman E, Healy E, et al. A family of vertebrate‐specific Polycombs encoded by the LCOR/LCORL genes balance PRC2 subtype activities. Mol Cell. 2018;70(3):408‐421.e8. [DOI] [PubMed] [Google Scholar]
- 30. Grijzenhout A, Godwin J, Koseki H, et al. Functional analysis of AEBP2, a PRC2 Polycomb protein, reveals a Trithorax phenotype in embryonic development and in ESCs. Development. 2016;143(15):2716‐2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hauri S, Comoglio F, Seimiya M, et al. A high‐density map for navigating the human Polycomb complexome. Cell Rep. 2016;17(2):583‐595. [DOI] [PubMed] [Google Scholar]
- 32. Jiao L, Shubbar M, Yang X, et al. A partially disordered region connects gene repression and activation functions of EZH2. Proc Natl Acad Sci U S A. 2020;117(29):16992‐17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mager J, Montgomery ND, de Villena FP‐M, Magnuson T. Genome imprinting regulated by the mouse Polycomb group protein EED. Nat Genet. 2003;33(4):502‐507. [DOI] [PubMed] [Google Scholar]
- 34. Koppens MAJ, Bounova G, Gargiulo G, et al. Deletion of Polycomb repressive complex 2 from mouse intestine causes loss of stem cells. Gastroenterology. 2016;151(4):684‐697.e12. [DOI] [PubMed] [Google Scholar]
- 35. Mirzamohammadi F, Papaioannou G, Inloes JB, et al. Polycomb repressive complex 2 regulates skeletal growth by suppressing Wnt and TGF‐β signalling. Nat Commun. 2016;7:12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mousavi K, Zare H, Wang AH, Sartorelli V. Polycomb protein Ezh1 promotes RNA polymerase II elongation. Mol Cell. 2012;45(2):255‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ai S, Peng Y, Li C, et al. EED orchestration of heart maturation through interaction with HDACs is H3K27me3‐independent. Elife. 2017;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Graffmann N, Brands J, Görgens A, et al. Age‐related increase of EED expression in early hematopoietic progenitor cells is associated with global increase of the histone modification H3K27me3. Stem Cells Dev. 2015;24(17):2018‐2031. [DOI] [PubMed] [Google Scholar]
- 39. Liu P‐P, Xu Y‐J, Dai S‐K, et al. Polycomb protein EED regulates neuronal differentiation through targeting SOX11 in hippocampal dentate gyrus. Stem Cell Reports. 2019;13(1):115‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang J, Yang L, Dong C, et al. EED‐mediated histone methylation is critical for CNS myelination and remyelination by inhibiting WNT, BMP, and senescence pathways. Sci Adv. 2020;6(33):eaaz6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang S‐F, Dai S‐K, Du H‐Z, et al. The epigenetic state of EED‐Gli3‐Gli1 regulatory axis controls embryonic cortical neurogenesis. Stem Cell Reports. 2022;17(9):2064‐2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79‐99. [DOI] [PubMed] [Google Scholar]
- 43. Jain BP, Pandey S. WD40 repeat proteins: Signalling scaffold with diverse functions. Protein J. 2018;37(5):391‐406. [DOI] [PubMed] [Google Scholar]
- 44. Han Z, Xing X, Hu M, Zhang Y, Liu P, Chai J. Structural basis of EZH2 recognition by EED. Structure. 2007;15(10):1306‐1315. [DOI] [PubMed] [Google Scholar]
- 45. Luo M. Chemical and biochemical perspectives of protein lysine methylation. Chem Rev. 2018;118(14):6656‐6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi Y, Wang X‐X, Zhuang Y‐W, Jiang Y, Melcher K, Xu HE. Structure of the PRC2 complex and application to drug discovery. Acta Pharmacol Sin. 2017;38(7):963‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiao L, Liu X. Structural basis of histone H3K27 trimethylation by an active polycomb repressive complex 2. Science. 2015;350(6258):aac4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu C, Bian C, Yang W, et al. Binding of different histone marks differentially regulates the activity and specificity of polycomb repressive complex 2 (PRC2). Proc Natl Acad Sci U S A. 2010;107(45):19266‐19271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sanulli S, Justin N, Teissandier A, et al. Jarid2 methylation via the PRC2 complex regulates H3K27me3 deposition during cell differentiation. Mol Cell. 2015;57(5):769‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poepsel S, Kasinath V, Nogales E. Cryo‐EM structures of PRC2 simultaneously engaged with two functionally distinct nucleosomes. Nat Struct Mol Biol. 2018;25(2):154‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Justin N, Zhang Y, Tarricone C, et al. Structural basis of oncogenic histone H3K27M inhibition of human polycomb repressive complex 2. Nat Commun. 2016;7:11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee C‐H, Yu J‐R, Kumar S, et al. Allosteric activation dictates PRC2 activity independent of its recruitment to chromatin. Mol Cell. 2018;70(3):422‐434.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moritz LE, Trievel RC. Structure, mechanism, and regulation of polycomb‐repressive complex 2. J Biol Chem. 2018;293(36):13805‐13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in Polycomb‐group silencing. Science. 2002;298(5595):1039‐1043. [DOI] [PubMed] [Google Scholar]
- 55. Martin C, Cao R, Zhang Y. Substrate preferences of the EZH2 histone methyltransferase complex. J Biol Chem. 2006;281(13):8365‐8370. [DOI] [PubMed] [Google Scholar]
- 56. Jain SU, Do TJ, Lund PJ, et al. PFA ependymoma‐associated protein EZHIP inhibits PRC2 activity through a H3 K27M‐like mechanism. Nat Commun. 2019;10(1):2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen S, Jiao L, Shubbar M, Yang X, Liu X. Unique structural platforms of Suz12 dictate distinct classes of PRC2 for chromatin binding. Mol Cell. 2018;69(5):840‐852.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Holoch D, Margueron R. Mechanisms regulating PRC2 recruitment and enzymatic activity. Trends Biochem Sci. 2017;42(7):531‐542. [DOI] [PubMed] [Google Scholar]
- 59. Lee C‐H, Holder M, Grau D, et al. Distinct stimulatory mechanisms regulate the catalytic activity of Polycomb repressive complex 2. Mol Cell. 2018;70(3):435‐448.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Perino M, van Mierlo G, Loh C, et al. Two functional axes of feedback‐enforced PRC2 recruitment in mouse embryonic stem cells. Stem Cell Reports. 2020;15(6):1287‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kasinath V, Faini M, Poepsel S, et al. Structures of human PRC2 with its cofactors AEBP2 and JARID2. Science. 2018;359(6378):940‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu J‐R, Lee C‐H, Oksuz O, Stafford JM, Reinberg D. PRC2 is high maintenance. Genes Dev. 2019;33(15–16):903‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peng JC, Valouev A, Swigut T, et al. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell. 2009;139(7):1290‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kasinath V, Beck C, Sauer P, et al. JARID2 and AEBP2 regulate PRC2 in the presence of H2AK119ub1 and other histone modifications. Science. 2021;371(6527):eabc3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang Q, Agius SC, Flanigan SF, et al. PALI1 facilitates DNA and nucleosome binding by PRC2 and triggers an allosteric activation of catalysis. Nat Commun. 2021;12(1):4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243‐269. [DOI] [PubMed] [Google Scholar]
- 67. Margueron R, Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet. 2010;11(4):285‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Laugesen A, Højfeldt JW, Helin K. Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol Cell. 2019;74(1):8‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aranda S, Mas G, Di Croce L. Regulation of gene transcription by Polycomb proteins. Sci Adv. 2015;1(11):e1500737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Blackledge NP, Klose RJ. The molecular principles of gene regulation by Polycomb repressive complexes. Nat Rev Mol Cell Biol. 2021;22(12):815‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Oksuz O, Narendra V, Lee C‐H, et al. Capturing the onset of PRC2‐mediated repressive domain formation. Mol Cell. 2018;70(6):1149‐1162.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chammas P, Mocavini I, Di Croce L. Engaging chromatin: PRC2 structure meets function. Br J Cancer. 2020;122(3):315‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19(10):1129‐1155. [DOI] [PubMed] [Google Scholar]
- 74. Karwacki‐Neisius V, Göke J, Osorno R, et al. Reduced Oct4 expression directs a robust pluripotent state with distinct signaling activity and increased enhancer occupancy by Oct4 and Nanog. Cell Stem Cell. 2013;12(5):531‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pereira CF, Piccolo FM, Tsubouchi T, et al. ESCs require PRC2 to direct the successful reprogramming of differentiated cells toward pluripotency. Cell Stem Cell. 2010;6(6):547‐556. [DOI] [PubMed] [Google Scholar]
- 76. Faust C, Schumacher A, Holdener B, Magnuson T. The EED mutation disrupts anterior mesoderm production in mice. Development. 1995;121(2):273‐285. [DOI] [PubMed] [Google Scholar]
- 77. Wang J, Mager J, Schnedier E, Magnuson T. The mouse PcG gene EED is required for Hox gene repression and extraembryonic development. Mamm Genome. 2002;13(9):493‐503. [DOI] [PubMed] [Google Scholar]
- 78. Morin‐Kensicki EM, Faust C, LaMantia C, Magnuson T. Cell and tissue requirements for the gene EED during mouse gastrulation and organogenesis. Genesis. 2001;31(4):142‐146. [DOI] [PubMed] [Google Scholar]
- 79. Shibata S, Yokota T, Wutz A. Synergy of EED and Tsix in the repression of Xist gene and X‐chromosome inactivation. EMBO J. 2008;27(13):1816‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chamberlain SJ, Yee D, Magnuson T. Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells. 2008;26(6):1496‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Leeb M, Pasini D, Novatchkova M, Jaritz M, Helin K, Wutz A. Polycomb complexes act redundantly to repress genomic repeats and genes. Genes Dev. 2010;24(3):265‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Obier N, Lin Q, Cauchy P, et al. Polycomb protein EED is required for silencing of pluripotency genes upon ESC differentiation. Stem Cell Rev Rep. 2015;11(1):50‐61. [DOI] [PubMed] [Google Scholar]
- 83. Galonska C, Ziller MJ, Karnik R, Meissner A. Ground state conditions induce rapid reorganization of core pluripotency factor binding before global epigenetic reprogramming. Cell Stem Cell. 2015;17(4):462‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. van Mierlo G, Dirks RAM, De Clerck L, et al. Integrative proteomic profiling reveals PRC2‐dependent epigenetic crosstalk maintains ground‐state pluripotency. Cell Stem Cell. 2019;24(1):123‐137.e8. [DOI] [PubMed] [Google Scholar]
- 85. Saitou M, Yamaji M. Primordial germ cells in mice. Cold Spring Harb Perspect Biol. 2012;4(11):a008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Seisenberger S, Andrews S, Krueger F, et al. The dynamics of genome‐wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell. 2012;48(6):849‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lesch BJ, Dokshin GA, Young RA, McCarrey JR, Page DC. A set of genes critical to development is epigenetically poised in mouse germ cells from fetal stages through completion of meiosis. Proc Natl Acad Sci U S A. 2013;110(40):16061‐16066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. de Napoles M, Nesterova T, Brockdorff N. Early loss of Xist RNA expression and inactive X chromosome associated chromatin modification in developing primordial germ cells. PLoS One. 2007;2(9):e860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mallol A, Guirola M, Payer B. PRDM14 controls X‐chromosomal and global epigenetic reprogramming of H3K27me3 in migrating mouse primordial germ cells. Epigenetics Chromatin. 2019;12(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Barau J, Teissandier A, Zamudio N, et al. The DNA methyltransferase DNMT3C protects male germ cells from transposon activity. Science. 2016;354(6314):909‐912. [DOI] [PubMed] [Google Scholar]
- 91. Mu W, Starmer J, Fedoriw AM, Yee D, Magnuson T. Repression of the soma‐specific transcriptome by Polycomb‐repressive complex 2 promotes male germ cell development. Genes Dev. 2014;28(18):2056‐2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lowe MG, Yen M‐R, Hsu F‐M, et al. EED is required for mouse primordial germ cell differentiation in the embryonic gonad. Dev Cell. 2022;57(12):1482‐1495.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Prokopuk L, Stringer JM, Hogg K, Elgass KD, Western PS. PRC2 is required for extensive reorganization of H3K27me3 during epigenetic reprogramming in mouse fetal germ cells. Epigenetics Chromatin. 2017;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Stringer JM, Forster SC, Qu Z, et al. Reduced PRC2 function alters male germline epigenetic programming and paternal inheritance. BMC Biol. 2018;16(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Prokopuk L, Stringer JM, White CR, et al. Loss of maternal EED results in postnatal overgrowth. Clin Epigenetics. 2018;10(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Prokopuk L, Jarred EG, Blücher RO, McLaughlin EA, Stringer JM, Western PS. An essential role for Polycomb repressive complex 2 in the mouse ovary. Reproduction. 2022;163(3):167‐182. [DOI] [PubMed] [Google Scholar]
- 97. Hirabayashi Y, Suzki N, Tsuboi M, et al. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron. 2009;63(5):600‐613. [DOI] [PubMed] [Google Scholar]
- 98. Yao B, Christian KM, He C, Jin P, Ming G‐L, Song H. Epigenetic mechanisms in neurogenesis. Nat Rev Neurosci. 2016;17(9):537‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Smigiel R, Biernacka A, Biela M, et al. Novel de novo mutation affecting two adjacent aminoacids in the EED gene in a patient with Weaver syndrome. J Hum Genet. 2018;63(4):517‐520. [DOI] [PubMed] [Google Scholar]
- 100. Song P‐P, Hu Y, Liu C‐M, et al. Embryonic ectoderm development protein is regulated by microRNAs in human neural tube defects. Am J Obstet Gynecol. 2011;204(6):544.e9‐544.e17. [DOI] [PubMed] [Google Scholar]
- 101. Shumacher A, Faust C, Magnuson T. Positional cloning of a global regulator of anterior‐posterior patterning in mice. Nature. 1996;383(6597):250‐253. [DOI] [PubMed] [Google Scholar]
- 102. Sun B, Chang E, Gerhartl A, Szele FG. Polycomb protein EED is required for neurogenesis and cortical injury activation in the subventricular zone. Cereb Cortex. 2018;28(4):1369‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yaghmaeian Salmani B, Monedero Cobeta I, Rakar J, et al. Evolutionarily conserved anterior expansion of the central nervous system promoted by a common PcG‐Hox program. Development. 2018;145(7):dev160747. [DOI] [PubMed] [Google Scholar]
- 104. Wang Y‐Y, Deng Y‐S, Dai S‐K, et al. Loss of microglial EED impairs synapse density, learning, and memory. Mol Psychiatry. 2022;27(7):2999‐3009. [DOI] [PubMed] [Google Scholar]
- 105. He A, Ma Q, Cao J, et al. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res. 2012;110(3):406‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Delgado‐Olguín P, Huang Y, Li X, et al. Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat Genet. 2012;44(3):343‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Montgomery RL, Davis CA, Potthoff MJ, et al. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21(14):1790‐1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. He A, Shen X, Ma Q, et al. PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes Dev. 2012;26(1):37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zaidi S, Choi M, Wakimoto H, et al. De novo mutations in histone‐modifying genes in congenital heart disease. Nature. 2013;498(7453):220‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shin AN, Dasgupta C, Zhang G, Seal K, Zhang L. Proteomic analysis of Endothelin‐1 targets in the regulation of cardiomyocyte proliferation. Curr Top Med Chem. 2017;17(15):1788‐1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Philipp S, Puchert M, Adam‐Klages S, et al. The Polycomb group protein EED couples TNF receptor 1 to neutral sphingomyelinase. Proc Natl Acad Sci U S A. 2010;107(3):1112‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yu W, Zhang F, Wang S, et al. Depletion of polycomb repressive complex 2 core component EED impairs fetal hematopoiesis. Cell Death Dis. 2017;8(4):e2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li Y, Ai S, Yu X, et al. Replication‐independent histone turnover underlines the epigenetic homeostasis in adult heart. Circ Res. 2019;125(2):198‐208. [DOI] [PubMed] [Google Scholar]
- 114. Liu J, Liu S, Han L, et al. LncRNA HBL1 is required for genome‐wide PRC2 occupancy and function in cardiogenesis from human pluripotent stem cells. Development. 2021;148(13):dev199628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003‐1007. [DOI] [PubMed] [Google Scholar]
- 116. Chiacchiera F, Rossi A, Jammula S, Zanotti M, Pasini D. PRC2 preserves intestinal progenitors and restricts secretory lineage commitment. EMBO J. 2016;35(21):2301‐2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lorzadeh A, Romero‐Wolf M, Goel A, Jadhav U. Epigenetic regulation of intestinal stem cells and disease: a balancing act of DNA and histone methylation. Gastroenterology. 2021;160(7):2267‐2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Vizán P, Beringer M, Di Croce L. Polycomb‐dependent control of cell fate in adult tissue. EMBO J. 2016;35(21):2268‐2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jadhav U, Nalapareddy K, Saxena M, et al. Acquired tissue‐specific promoter bivalency is a basis for PRC2 necessity in adult cells. Cell. 2016;165(6):1389‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jadhav U, Cavazza A, Banerjee KK, et al. Extensive recovery of embryonic enhancer and gene memory stored in hypomethylated enhancer DNA. Mol Cell. 2019;74(3):542‐554.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jadhav U, Manieri E, Nalapareddy K, et al. Replicational dilution of H3K27me3 in mammalian cells and the role of poised promoters. Mol Cell. 2020;78(1):141‐151.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fuchs E. Scratching the surface of skin development. Nature. 2007;445(7130):834‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang J, Bardot ES, Ezhkova E. Epigenetic regulation of skin: focus on the Polycomb complex. Cell Mol Life Sci. 2012;69(13):2161‐2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Watt FM, Lo Celso C, Silva‐Vargas V. Epidermal stem cells: an update. Curr Opin Genet Dev. 2006;16(5):518‐524. [DOI] [PubMed] [Google Scholar]
- 125. Lin Z, Jin S, Chen J, et al. Murine interfollicular epidermal differentiation is gradualistic with GRHL3 controlling progression from stem to transition cell states. Nat Commun. 2020;11(1):5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fu X, Sun X. Can hematopoietic stem cells be an alternative source for skin regeneration? Ageing Res Rev. 2009;8(3):244‐249. [DOI] [PubMed] [Google Scholar]
- 127. Ezhkova E, Pasolli HA, Parker JS, et al. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue‐specific stem cells. Cell. 2009;136(6):1122‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shaw T, Martin P. Epigenetic reprogramming during wound healing: loss of polycomb‐mediated silencing may enable upregulation of repair genes. EMBO Rep. 2009;10(8):881‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Dauber KL, Perdigoto CN, Valdes VJ, Santoriello FJ, Cohen I, Ezhkova E. Dissecting the roles of Polycomb repressive complex 2 subunits in the control of skin development. J Invest Dermatol. 2016;136(8):1647‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Perdigoto CN, Dauber KL, Bar C, et al. Polycomb‐mediated repression and sonic hedgehog signaling interact to regulate Merkel cell specification during skin development. PLoS Genet. 2016;12(7):e1006151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cohen I, Bar C, Liu H, et al. Polycomb complexes redundantly maintain epidermal stem cell identity during development. Genes Dev. 2021;35(5–6):354‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Flora P, Li M‐Y, Galbo PM, Astorkia M, Zheng D, Ezhkova E. Polycomb repressive complex 2 in adult hair follicle stem cells is dispensable for hair regeneration. PLoS Genet. 2021;17(12):e1009948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Li M‐Y, Flora P, Pu H, et al. UV‐induced reduction in Polycomb repression promotes epidermal pigmentation. Dev Cell. 2021;56(18):2547‐2561.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Abedi M, Greer DA, Foster BM, et al. Critical variables in the conversion of marrow cells to skeletal muscle. Blood. 2005;106(4):1488‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Harrison DE, Zhong RK, Jordan CT, Lemischka IR, Astle CM. Relative to adult marrow, fetal liver repopulates nearly five times more effectively long‐term than short‐term. Exp Hematol. 1997;25(4):293‐297. [PubMed] [Google Scholar]
- 137. Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase‐specific engraftment defect. J Clin Invest. 2006;116(10):2808‐2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Xie H, Xu J, Hsu JH, et al. Polycomb repressive complex 2 regulates normal hematopoietic stem cell function in a developmental‐stage‐specific manner. Cell Stem Cell. 2014;14(1):68‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804‐1808. [DOI] [PubMed] [Google Scholar]
- 140. Essers MAG, Offner S, Blanco‐Bose WE, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904‐908. [DOI] [PubMed] [Google Scholar]
- 141. Lessard J, Schumacher A, Thorsteinsdottir U, van Lohuizen M, Magnuson T, Sauvageau G. Functional antagonism of the Polycomb‐group genes EED and Bmi1 in hemopoietic cell proliferation. Genes Dev. 1999;13(20):2691‐2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Richie ER, Schumacher A, Angel JM, Holloway M, Rinchik EM, Magnuson T. The Polycomb‐group gene EED regulates thymocyte differentiation and suppresses the development of carcinogen‐induced T‐cell lymphomas. Oncogene. 2002;21(2):299‐306. [DOI] [PubMed] [Google Scholar]
- 143. Ueda T, Nakata Y, Nagamachi A, et al. Propagation of trimethylated H3K27 regulated by polycomb protein EED is required for embryogenesis, hematopoietic maintenance, and tumor suppression. Proc Natl Acad Sci U S A. 2016;113(37):10370‐10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Majewski IJ, Ritchie ME, Phipson B, et al. Opposing roles of polycomb repressive complexes in hematopoietic stem and progenitor cells. Blood. 2010;116(5):731‐739. [DOI] [PubMed] [Google Scholar]
- 145. Vu TH, Shipley JM, Bergers G, et al. MMP‐9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Caplan AI. Bone development. Ciba Found Symp. 1988;136:3‐21. [DOI] [PubMed] [Google Scholar]
- 147. Lee S‐Y, Abel ED, Long F. Glucose metabolism induced by Bmp signaling is essential for murine skeletal development. Nat Commun. 2018;9(1):4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Erickson CA, Reedy MV. Neural crest development: the interplay between morphogenesis and cell differentiation. Curr Top Dev Biol. 1998;40:177‐209. [DOI] [PubMed] [Google Scholar]
- 149. Cohen ASA, Tuysuz B, Shen Y, Bhalla SK, Jones SJM, Gibson WT. A novel mutation in EED associated with overgrowth. J Hum Genet. 2015;60(6):339‐342. [DOI] [PubMed] [Google Scholar]
- 150. Cohen AS, Gibson WT. EED‐associated overgrowth in a second male patient. J Hum Genet. 2016;61(9):831‐834. [DOI] [PubMed] [Google Scholar]
- 151. Cooney E, Bi W, Schlesinger AE, Vinson S, Potocki L. Novel EED mutation in patient with Weaver syndrome. Am J Med Genet A. 2017;173(2):541‐545. [DOI] [PubMed] [Google Scholar]
- 152. Imagawa E, Higashimoto K, Sakai Y, et al. Mutations in genes encoding polycomb repressive complex 2 subunits cause Weaver syndrome. Hum Mutat. 2017;38(6):637‐648. [DOI] [PubMed] [Google Scholar]
- 153. Kim SY, Paylor SW, Magnuson T, Schumacher A. Juxtaposed Polycomb complexes co‐regulate vertebral identity. Development. 2006;133(24):4957‐4968. [DOI] [PubMed] [Google Scholar]
- 154. Lian W‐S, Wu R‐W, Ko J‐Y, et al. Histone H3K27 demethylase UTX compromises articular chondrocyte anabolism and aggravates osteoarthritic degeneration. Cell Death Dis. 2022;13(6):538. [DOI] [PMC free article] [PubMed] [Google Scholar]


