Abstract
Within the last year, four randomised-controlled clinical trials (RCTs) have been published comparing intravenous thrombolysis (IVT) with tenecteplase and alteplase in acute ischaemic stroke (AIS) patients with a non-inferiority design for three of them. An expedited recommendation process was initiated by the European Stroke Organisation (ESO) and conducted according to ESO standard operating procedure based on the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework. We identified three relevant Population, Intervention, Comparator, Outcome (PICO) questions, performed systematic reviews of the literature and meta-analyses, assessed the quality of the available evidence, and wrote evidence-based recommendations. Expert consensus statements were provided if insufficient evidence was available to provide recommendations based on the GRADE approach. For patients with AIS of <4.5 h duration who are eligible for IVT, tenecteplase 0.25 mg/kg can be used as a safe and effective alternative to alteplase 0.9 mg/kg (moderate evidence, strong recommendation). For patients with AIS of <4.5 h duration who are eligible for IVT, we recommend against using tenecteplase at a dose of 0.40 mg/kg (low evidence, strong recommendation). For patients with AIS of <4.5 h duration with prehospital management with a mobile stroke unit who are eligible for IVT, we suggest tenecteplase 0.25 mg/kg over alteplase 0.90 mg/kg (low evidence, weak recommendation). For patients with large vessel occlusion (LVO) AIS of <4.5 h duration who are eligible for IVT, we recommend tenecteplase 0.25 mg/kg over alteplase 0.9 mg/kg (moderate evidence, strong recommendation). For patients with AIS on awakening from sleep or AIS of unknown onset who are selected with non-contrast CT, we recommend against IVT with tenecteplase 0.25 mg/kg (low evidence, strong recommendation). Expert consensus statements are also provided. Tenecteplase 0.25 mg/kg may be favoured over alteplase 0.9 mg/kg for patients with AIS of <4.5 h duration in view of comparable safety and efficacy data and easier administration. For patients with LVO AIS of <4.5 h duration who are IVT-eligible, IVT with tenecteplase 0.25 mg/kg is preferable over skipping IVT before MT, even in the setting of a direct admission to a thrombectomy-capable centre. IVT with tenecteplase 0.25 mg/kg may be a reasonable alternative to alteplase 0.9 mg/kg for patients with AIS on awakening from sleep or AIS of unknown onset and who are IVT-eligible after selection with advanced imaging.
Keywords: Intravenous thrombolysis, acute ischaemic stroke, tenecteplase, recommendations, European Stroke Organisation, large vessel occlusion, wake-up stroke, extended time window
Introduction
Intravenous thrombolysis (IVT) with alteplase is the only approved fibrinolytic treatment for patients with acute ischaemic stroke (AIS).1,2 Alteplase at the dose of 0.9 mg/kg (maximum 90 mg) is administrated as a 10% bolus followed by a 1-h continuous infusion. Tenecteplase is a genetically modified form of alteplase with an increased resistance to plasminogen activator inhibitor 1, a greater fibrin specificity and a longer half-life, allowing for single bolus administration.3,4 Compared to alteplase, IVT with tenecteplase resulted in similar mortality and led to a reduction of systemic bleeding in patients with acute myocardial infarction. 5 Hence, tenecteplase is the recommended first-line thrombolytic agent when coronary intervention is not available in a timely fashion. 6 In animal models of AIS, tenecteplase seems to be associated with more rapid and complete reperfusion than alteplase, with less intracranial haemorrhages.7,8 After several phase 2 trials in AIS patients testing doses ranging from 0.10 to 0.50 mg/kg (maximum bolus dose of 10–50 mg), the doses of 0.10–0.40 mg/kg were considered to have the more advantageous profile of safety.9,10 Easier IVT administration with a single tenecteplase bolus is advantageous in the setting of AIS, being less time consuming in an emergency setting, potentially reducing door-to-needle time, and facilitating the organisation of intra- and inter-hospital transfers for patients eligible for mechanical thrombectomy (MT).11,12 The combination of these preliminary efficacy and safety data with its practical advantages, has led to the formal comparison of tenecteplase and alteplase in randomised-controlled clinical trials (RCT) for AIS patients eligible for IVT.
Based on the analysis of available RCTs, European Stroke Organisation (ESO) guidelines in 2021, suggested IVT with alteplase over tenecteplase for patients with AIS within 4.5 h of stroke onset and not eligible for MT.2,13–15 For patients with an identified large vessel occlusion (LVO), ESO guidelines analysed data from available RCTs and a study level meta-analysis. 2 They suggested IVT with 0.25 mg/kg tenecteplase over alteplase in patients with AIS within 4.5 h of stroke onset and LVO who were candidates for mechanical thrombectomy (MT).2,16–18
Within the last year, 4 RCTs comparing IVT with tenecteplase and alteplase have been published, with three of them using non-inferiority designs.19–22 In light of these new data, we sought to update our previous ESO guidelines in order to reconsider the place of intravenous tenecteplase in patients with AIS eligible for IVT.
Methods
This expedited recommendation was initiated by ESO and prepared according to the ESO standard operating procedure, 23 which is based on the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system. 24 The ESO Guideline Board and ESO Executive Committee reviewed the intellectual and financial disclosures of all module working group (MWG) members (Supplemental Table 1) and approved the composition of the group, which was chaired by Sonia Alamowitch and Georgios Tsivgoulis. The MWG was composed of nine voting members and two fellows with non-voting rights.
The steps undertaken by the MWG are summarised as follows:
1. The single topic of whether IVT with tenecteplase may be administered instead of the current standard of care (IVT with alteplase at a dose of 0.9 mg/kg) in patients with acute ischaemic stroke (AIS) was considered in this expedited recommendation. However, the MWG deemed it to be important to consider separately the role of IVT with tenecteplase for all AIS patients and, specifically, in patients with AIS due to large vessel occlusion (LVO). This approach was justified by the results of a recent pairwise meta-analysis 25 indicating the superiority of IVT with tenecteplase over alteplase in this specific stroke subgroup and recent ESO guidelines 2 on IVT for AIS that provide different recommendations for the use of tenecteplase in ‘unselected’ AIS (i.e. not selected based on vessel occlusion status or findings of advanced neuroimaging) and in LVO patients. Furthermore, AIS patients treated with tenecteplase at a dose of 0.25 mg/kg and at a dose of 0.4 mg/kg were separately evaluated in comparison to the current standard of care (IVT with alteplase at a dose of 0.9 mg/kg).
2. A list of relevant outcomes was produced and rated by each MWG member using secret ballot voting on a scale from 1 (not important) to 9 (extremely important). 24 The mean value for each outcome is reported below.
According to GRADE, five outcomes were considered to be of critical importance (mean score of 7–9) for patients with AIS:
Excellent functional outcome,26–28 defined as 90-day modified Rankin Scale (mRS) scores of 0–1: 8.7
Good functional outcome,26–28 defined as 90-day mRS scores of 0–2: 7.9
Reduced disability5,6 (⩾1-point reduction in mRS-score at 90 days): 7.8
Symptomatic intracranial haemorrhage (sICH) at 24–48 h: 7.7
Mortality at 90 days: 7.6
The following 9 other outcomes were considered to be of importance, but not critical (mean score 6–4) for making a decision for patients with AIS:
Major neurological improvement (according to definitions used in individual trials) at 24-–72 h: 6.2
Reperfusion at 24 h: 6.2
Final infarct volume at 24 h: 5.9
Quality of life metrics [measured with the EuroQol-5 Dimension (EQ5D) at 90 days]: 5.8
Ischaemic core growth within the first 24 h: 5.6
Door-to-needle time: 5.4
Any ICH: 5.1
Onset-to-treatment time: 5.0
Extracranial bleeding: 4.7
According to GRADE, five outcomes were considered to be of critical importance (mean score of 7–9) for the subgroup of patients with LVO:
Good functional outcome at 90 days: 8.3
Excellent functional outcome at 90 days: 8.2
Reduced disability at 90 days: 7.8
sICH at 24–48 h: 7.7
Mortality at 90 days: 7.6
A total of 12 other outcomes were considered to be of importance, but not critical (mean score 6–4) for making a decision for the subgroup of patients with LVO:
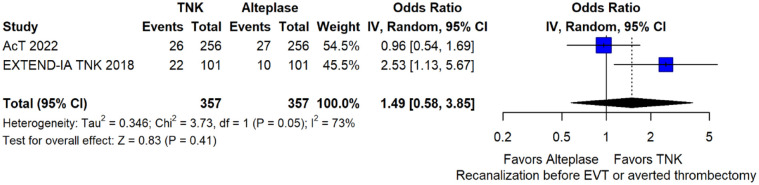
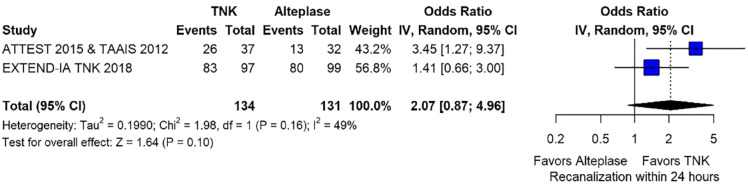
Recanalisation29,30 at the end of Mechanical Thrombectomy (MT) or at 24 h defined as modified Treatment In Cerebral Ischaemia (mTICI) score ⩾2b: 6.8
Recanalisation29,30 before MT at first angiographic acquisition [(mTICI) score ⩾2b] or averted MT: 6.8
Reperfusion at 24 h: 6.3
Major neurological improvement at 24–72 h: 6.2
Final infarct volume at 24 h: 6.2
Quality of life metrics: 5.9
Ischaemic core growth within the 24 h: 5.7
Door-to-needle time: 5.8
Needle to groin puncture time: 5.6
Any ICH: 5.1
Onset-to-treatment time: 5.1
Extracranial bleeding: 4.7
Based on this vote, excellent functional outcome (mRS 0–1 at 90 days) was defined as the outcome of highest priority for all AIS and was considered first. Moreover, good functional outcome (mRS 0–2 at 90 days) was defined as the outcome of highest priority for the PICO question related to LVO patients. Unless specified otherwise, reduced disability26,27 corresponded to a reduction of at least one point in the mRS score at 90 days across all mRS grades (‘shift analysis’). sICH was defined according to each study’s original criterion. In the case of limited data for the outcomes of highest importance, outcomes of lesser importance were also considered.
3. The MWG formulated a list of Population, Intervention, Comparator, Outcome (PICO) questions, which were reviewed and subsequently approved by external reviewers and members of the ESO Guideline Board and ESO Executive Committee.
4. The recommendation for the PICO questions were based on a systematic review of RCTs comparing IVT with tenecteplase to IVT with alteplase. The literature research used the three following bibliographic databases (Pubmed, Embase, Cochrane Library) and was conducted up to September 2022. We have also included results of RCTs presented at international conferences that were not published at the time of preparation of this expedited recommendation.
5. The risk of bias in each RCT was assessed using the Cochrane’s collaboration Risk of Bias 2 (RoB2) tool. 31
6. Whenever appropriate, random-effects meta-analyses were conducted using R software version 3.5.0 (metafor package). 32 Results were summarised as odds ratio (ORs), common Odds Ratios (cOR), or risk difference (RD) with their 95% confidence intervals (CIs).27,28 Time metrics and infarct volumes were evaluated using differences of medians or means (MD) with corresponding interquartile ranges (IQRs) or standard deviations (SD), respectively, as provided in individual studies. 28 The pooled mean difference is reported with corresponding 95%CI. 27 Heterogeneity was classified as low (I 2 <30%), moderate (I2 ⩾ 30%), substantial (I2 ⩾ 50%), or considerable (I2 ⩾ 75%).27,28
7. Before statistical analyses were conducted the MWG decided that the assessment of non-inferiority would be based on the absolute difference (RD) in the proportions of AIS patients achieving excellent functional outcome (mRS 0–1) between the two treatment groups (IVT with tenecteplase and IVT with alteplase, the latter being the reference group). An absolute non-inferiority margin was chosen via secret ballot voting. The minimal and maximal values for a non-inferiority margin advocated by MWG members were 1.3% and 3.0%, respectively. A majority (7/9) of MWG members voted for a margin of 3.0% that was the most stringent absolute non-inferiority margin selected among all published RCTs comparing the safety and efficacy of IVT with tenecteplase to IVT with alteplase in AIS patients. 22 Another argument for the selection of this specific non-inferiority margin was based on the findings of an individual participant data meta-analysis (IPD-MA) of RCTs comparing IVT with alteplase and placebo in AIS patients. 1 The corresponding estimate of weighted absolute effect for excellent functional outcome with alteplase compared to placebo for the IPD-MA population was 8.5% (95% CI 6%–11%) based on the proportions of patients treated within 0–4.5 h from symptom onset. 1 The absolute non-inferiority margin of 3% was chosen since it would preserve at least half of the conservative (lower 95%CI limit) estimate of alteplase effect.33,34 Two out of nine MWG members voted for a margin of 1.3% which corresponds to the median minimal clinically important difference in a survey of U.S. stroke neurologists. 35 Therefore, we prespecified that for the present recommendation, non-inferiority would be met for excellent functional outcome in all AIS patients if the lower 95% CI boundary of the random-effects pooled RD was superior or equal to −3.0%. An absolute non-inferiority margin of 1.3% was also assessed for AIS patients as a secondary analysis. For LVO patients, the MWG decided that the assessment of non-inferiority would be based on the RD in the proportions of LVO patients achieving good functional outcome (mRS 0–2) between the two treatment groups (IVT with tenecteplase and IVT with alteplase, the latter being the reference group), since good functional outcome was graded as the one with the highest clinical importance in the LVO subgroup. A non-inferiority margin of 1.3% was unanimously chosen by all MWG members for reasons of consistency with a previous ESO expedited recommendation that evaluated the comparative safety and efficacy of direct MT compared to bridging therapy (IVT and MT) in LVO patients. 27 In this recommendation a non-inferiority margin of 1.3% was selected for the RD in good functional outcome between the two treatment groups (direct MT and bridging therapy). 27 A non-inferiority margin of 3.0% was also assessed for LVO patients as a secondary analysis. For all reported analyses, random-effects pooled RD was calculated using the DerSimonian and Laird method. 36 No p-value for non-inferiority was computed.
8. The results of data analyses were imported into the GRADEpro Guideline Development Tool (McMaster University, 2015; developed by Evidence Prime, Inc.). For each PICO question and each outcome, the risk of bias was assessed and the quality of evidence was rated as high, moderate, low or very low based on the type of available evidence (randomised) and considerations on inconsistency of results, indirectness of evidence, imprecision of results, and risk of bias. 24 GRADE evidence profiles/summary of findings tables were generated using GRADEPro.
9. As per ESO standard operating procedures, 23 each PICO question was addressed by writing up to three distinct paragraphs. First, a paragraph named ‘Analysis of current evidence’, in which the results of the dedicated RCTs were summarised and briefly discussed. Where no RCT was available, this paragraph described results of systematic reviews of non-randomised studies. At the end of the first paragraph, an evidence-based recommendation was provided, based on the GRADE methodology. The direction, the strength and the formulation of the recommendation were determined according to the GRADE evidence profiles and the ESO standard operating procedure. Second, an ‘Additional information’ paragraph could be added to provide more details on randomised trials mentioned in the first paragraph, to summarise results of observational studies, or to provide information on ongoing or future trials. Third, an ‘Expert consensus statement’ paragraph was added whenever the PICO group deemed that the available evidence was insufficient to provide evidence-based recommendations for situations in which practical guidance is needed for routine clinical practice. In that case, a pragmatic suggestion was provided, together with the results of the votes of all MWG members. Importantly, the suggestions provided in this paragraph should not be mistaken as evidence-based recommendations.
10. This Expedited Recommendation document was subsequently reviewed several times by all MWG members, and iteratively modified until a consensus was reached. Finally, the document was reviewed and approved by external reviewers and members of the ESO Guideline Board and Executive Committee.
Results
PICO 1 AIS patients <4.5 h
1.1 For patients with acute ischaemic stroke of <4.5 h duration, does intravenous thrombolysis with tenecteplase 0.25 mg/kg compared with intravenous thrombolysis with alteplase 0.90 mg/kg lead to:
(a) a non-inferior proportion of patients with excellent functional outcome (mRS scores of 0–1) at 90 days?
(b) non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, good functional outcome defined by mRS 0–2 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)?
c) a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)?
(d) a reduction in key time metrics (onset-to-treatment time, door-to-needle time)?
(e) an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth within the first 24 h)?
Analysis of current evidence
The literature search identified seven published RCTs addressing this PICO question.
TNK-S2B (Study of Tenecteplase in Acute Ischaemic Stroke) was a double-blind, phase 2b/3 RCT that randomised 112 AIS patients in the United States within 3 h from symptom onset in 4 treatment arms (tenecteplase 0.10, 0.25, 0.40 mg/kg or alteplase 0.9 mg/kg). 13 During phase 2b, the trial explored the optimal dose of tenecteplase to carry forward, based on a composite outcome measure that included sICH within 24 h and functional outcome at 3 months. The 0.40 mg/kg dose was discarded as inferior after only 73 patients were randomised, but the selection process was still unable to distinguish between 0.10 and 0.25 mg/kg as a propitious dose at the time the trial was stopped. There was not a statistically significant difference in 3-month outcomes between the 0.10 and 0.25 mg/kg tenecteplase groups and alteplase. sICH rates were 0%, 6.5% and 15.8% for the 0.10, 0.25 and 0.40 mg/kg tenecteplase groups respectively.
TAAIS (Tenecteplase versus Alteplase for Acute Ischaemic Stroke) was a phase 2b RCT with prospective, randomised, open-label, blinded end-point (PROBE) design that randomised 75 AIS patients in Australia presenting within 6 h from symptom onset, with evidence of vessel occlusion on computed tomographic (CT) angiography and a perfusion lesion at least 20% greater than the infarct core on CT perfusion imaging at baseline in three treatment arms (tenecteplase 0.10, 0.25 mg/kg or alteplase 0.9 mg/kg). 16 Patients that were offered MT were excluded from this study. The co-primary endpoints included the proportion of the perfusion lesion that was reperfused at 24 h on perfusion-weighted magnetic resonance imaging (MRI), and the extent of clinical improvement at 24 h as assessed on NIHSS scores. Together, the two tenecteplase groups had greater reperfusion and neurological improvement at 24 h than the alteplase group. There were no significant between-group differences in intracranial bleeding or other serious adverse events. The 0.25 mg/kg dose of tenecteplase was superior to both the 0.10 mg/kg dose and alteplase for all efficacy outcomes including excellent functional outcome at 3 months (72% with 0.25 mg/kg tenecteplase dose vs 40% with alteplase; p = 0.02). Yet, this study was limited due to the inclusion of a modest number of patients in each arm.
ATTEST (Alteplase-Tenecteplase Trial Evaluation for Stroke Thrombolysis) was a phase 2 RCT with PROBE design that randomised 104 patients with supratentorial AIS in the United Kingdom within 4.5 h from symptom onset in two treatment arms: tenecteplase 0.25 mg/kg or alteplase 0.9 mg/kg. 15 Almost three-quarters of the included patients had an arterial occlusion on CT angiography; however, MT was not performed. The primary endpoint was the percentage of penumbra salvaged (CT perfusion-defined penumbra volume at baseline minus CT infarct volume at 24–48 h). Clinical and radiological efficacy and safety endpoints did not differ between the tenecteplase and alteplase groups.
EXTEND-IA TNK (Tenecteplase versus Alteplase before Endovascular Therapy for Ischaemic Stroke) was a phase 2 RCT with PROBE design that randomised 202 LVO patients who were eligible to undergo mechanical thrombectomy (MT) within 4.5 h from symptom onset in Australia and New Zealand in two treatment arms: tenecteplase 0.25 mg/kg or alteplase 0.9 mg/kg. 17 The primary endpoint was reperfusion of greater than 50% in the involved ischaemic territory or absence of retrievable thrombus at the time of the initial angiographic assessment. The primary endpoint occurred in 22% of the patients treated with tenecteplase versus 10% of those treated with alteplase (incidence rate ratio, 2.2; 95%CI: 1.1–4.4; p = 0.002 for non-inferiority; p = 0.03 for superiority). Tenecteplase resulted in reduced disability at 90-days (adjusted common odds ratio for ⩾ 1-point decrease across all mRS-scores: 1.7; 95%CI: 1.0–2.8; p = 0.04). sICH occurred in 1% of the patients in each group. In summary, tenecteplase before MT was associated with a higher incidence of reperfusion and reduced disability at 90 days among LVO patients with AIS treated within 4.5 h after symptom onset.
TRACE (Tenecteplase Reperfusion Therapy in Acute Ischaemic Cerebrovascular Events) was a phase 2 RCT with PROBE design that randomised 236 AIS patients in China within 3 h from symptom onset in 4 treatment arms (tenecteplase 0.10, 0.25, 0.32 mg/kg or alteplase 0.9 mg/kg). 19 The primary endpoint was sICH within 24 h. The rates of sICH were 5.0%, 0%, 3.3% and 1.7% in the tenecteplase 0.10 mg/kg group, 0.25 mg/kg group, 0.32 mg/kg group and in the alteplase group, respectively. There were no significant between-group differences in severe adverse events and functional outcomes.
TASTE-A (Tenecteplase Versus Alteplase for Stroke Thrombolysis Evaluation Trial in the Ambulance) was a phase 2 RCT with PROBE design that randomised 104 AIS patients in mobile stroke units (MSUs) in Australia within 4.5 h from symptom onset in two treatment arms: tenecteplase 0.25 mg/kg or alteplase 0.9 mg/kg. 20 The primary endpoint was the volume of the perfusion lesion on arrival at hospital, assessed by CT-perfusion imaging. On hospital arrival the perfusion lesion volume was significantly smaller in the tenecteplase arm [median 12 mL (IQR 3–28)] compared with alteplase [35 mL (IQR 18–76)]; adjusted incidence rate ratio 0.55 (95%CI: 0.37–0.81; p = 0.003)]. At 90 days, mRS-scores of 5 or 6 were reported in 15% and 20% of patients allocated to tenecteplase and alteplase respectively (adjusted OR: 0.70, 95%CI: 0.23–2.16; p = 0.54). Additionally, there were no significant differences among patients treated with tenecteplase versus alteplase on any functional outcome at 90 days.
AcT (Alteplase compared to Tenecteplase) was a phase 3, registry-based, non-inferiority RCT with PROBE design that randomised 1600 AIS patients in Canada within 4.5 h from symptom onset in two treatment arms: tenecteplase 0.25 mg/kg or alteplase 0.9 mg/kg. 21 The primary endpoint was the proportion of patients with excellent functional outcome (mRS 0–1), measured as close to 90 days after randomisation as possible (median follow-up duration of 97 days), with allowance of follow-up evaluations being up to 120 days after randomisation. Non-inferiority would have been met if the lower 95% CI of the difference in the proportion of patients with excellent functional outcome between the tenecteplase and alteplase groups was more than –5%. The rates of primary endpoint were 36.9% in the tenecteplase group and 34.8% in the alteplase group [unadjusted risk difference 2.1% (95%CI: –2.6 to 6.9)], meeting the pre-specified non-inferiority threshold. In safety analyses, the rates of sICH were 3.4% and 3.2% for tenecteplase and alteplase, respectively.
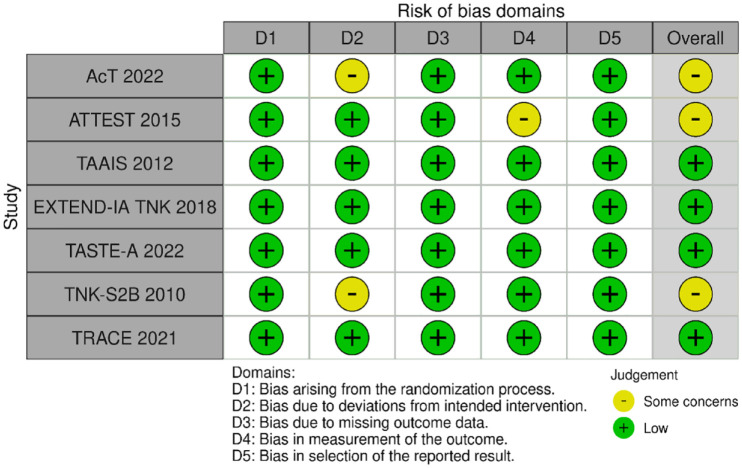
MWG assessment of the risk of bias in each RCT according to the Cochrane RoB-2 tool with regard to excellent functional outcome at 90 days is presented in Figure 1. All studies were considered to be at overall low risk of bias, except for the following: (i) AcT 21 and TNK-S2B, 13 which presented some concerns due to small deviations from intended interventions; (ii) ATTEST, 15 due to concerns regarding the assessment of endpoint, since masking to treatment allocation for clinical endpoints’ assessment could not be guaranteed.
Figure 1.
Risk of bias in each randomised controlled clinical trial of IVT with tenecteplase at a dose of 0.25 mg/kg versus IVT with alteplase for AIS patients, with regards to excellent functional outcome at 90 days.
Small deviations from intended interventions were noticed: (i) in the AcT trial, where 6/806 patients randomised to the tenecteplase – group and 9/771 randomised to the alteplase – group did not receive the assigned treatment, and (ii) in the TNK-S2B trial, where one patient who was randomised to the alteplase – group received 0.25 mg/kg tenecteplase and one patient who was randomised to 0.25 mg/kg tenecteplase received 0.7 mg/kg tenecteplase.
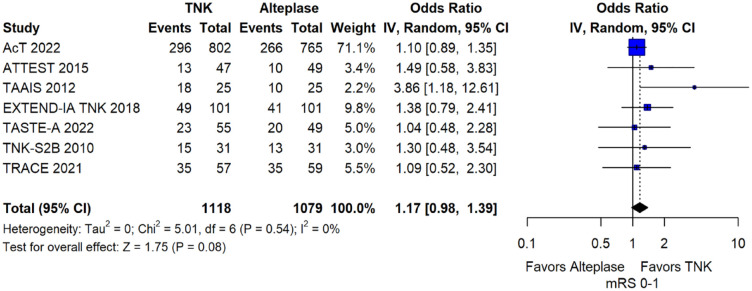
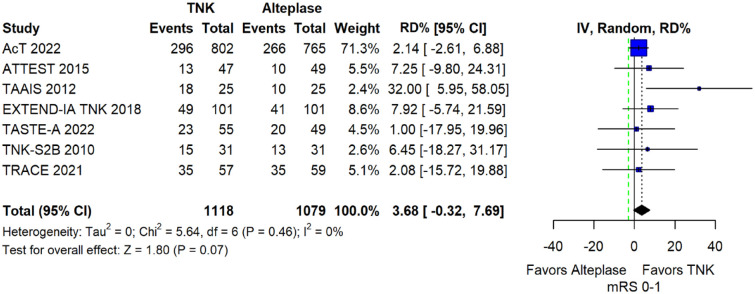
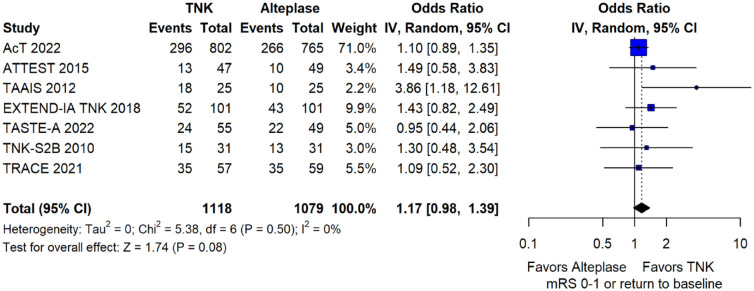
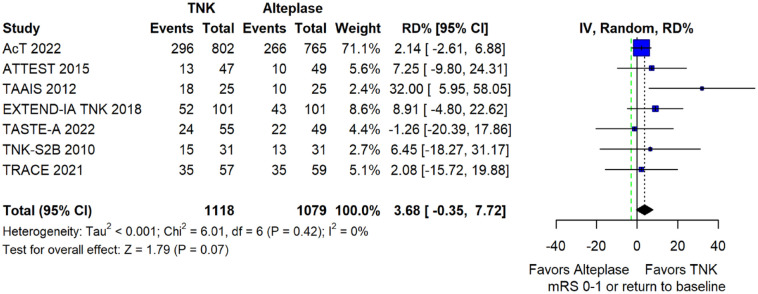
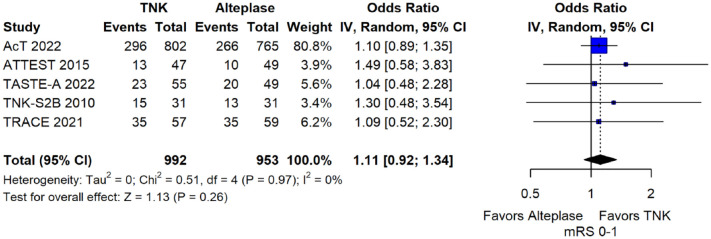
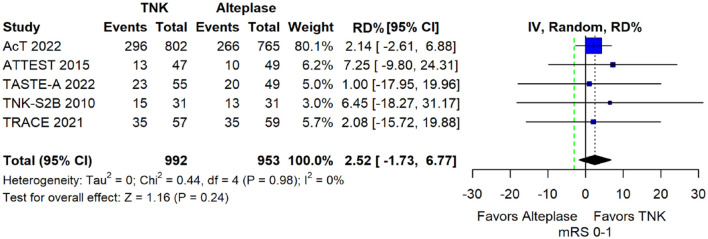
We conducted a study-level random-effects meta-analysis of the seven RCTs comparing IVT with tenecteplase 0.25 mg/kg versus IVT with alteplase 0.9 mg/kg, comprising a total of 2197 AIS patients. Compared to patients randomised to IVT with alteplase the pooled unadjusted OR for excellent functional outcome in patients randomised to IVT with tenecteplase was 1.17 (95%CI: 0.98–1.39; p = 0.08; I2 = 0%; Figure 2). The corresponding risk difference was 3.68% (95%CI: −0.32% to 7.69%; p = 0.07; I2 = 0%; Figure 3). Therefore, non-inferiority was met for the excellent functional outcome based on our pre-specified 3% margin. Importantly though, non-inferiority was also met based on the minimum clinically important difference of 1.3% proposed by some MWG members. Similar results were obtained when we conducted a sensitivity analysis for excellent functional outcome after additional inclusion of all patients returning to baseline mRS (Figures 4 and 5). A second sensitivity analysis was performed by excluding the RCTs that used additional selection criteria: (i) patients in TAAIS 16 were selected on the basis of visible arterial occlusion on CT angiography and the presence of certain CT perfusion mismatch and (ii) patients in EXTEND-IA TNK 17 were selected based on contrast angiography and eligibility of mechanical thrombectomy. Similar results were also obtained in this sensitivity analysis: pooled unadjusted OR for excellent functional outcome 1.11 (95%CI: 0.92–1.34; p = 0.26; I2 = 0%; Figure 6); corresponding risk difference 2.52% (95%CI: −1.73% to 6.77%; p = 0.24; I2 = 0%; Figure 7). In all sensitivity analyses, non-inferiority was met for the excellent functional outcome based on our pre-specified 3% margin.
Figure 2.
Excellent functional outcome (mRS 0–1 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects metaanalysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 3.
Pooled risk difference (in percent) for excellent functional outcome (mRS 0–1 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
Figure 4.
Sensitivity analysis for excellent functional outcome (mRS 0–1 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after additional inclusion of all patients returning to baseline mRS (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 5.
Sensitivity analysis for pooled risk difference (in percent) for excellent functional outcome (mRS 0–1 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after additional inclusion of all patients returning to baseline mRS (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
Figure 6.
Sensitivity analysis for excellent functional outcome (mRS 0–1 at 90 days) in unselected patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after excluding the randomised controlled clinical trials that used additional selection criteria (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 7.
Sensitivity analysis for pooled risk difference (in percent) for excellent functional outcome (mRS 0–1 at 90 days) in unselected patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after excluding the randomised controlled clinical trials that used additional selection criteria (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
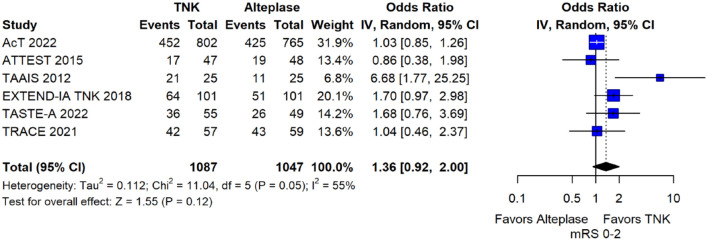
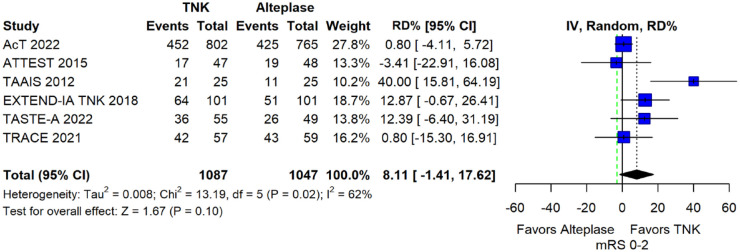
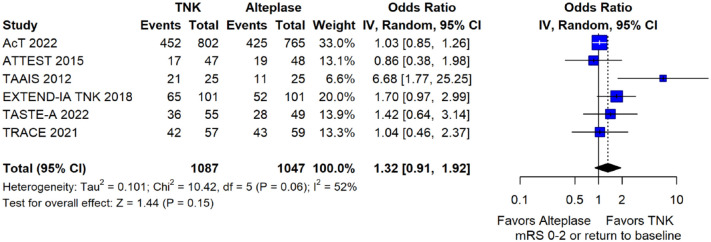
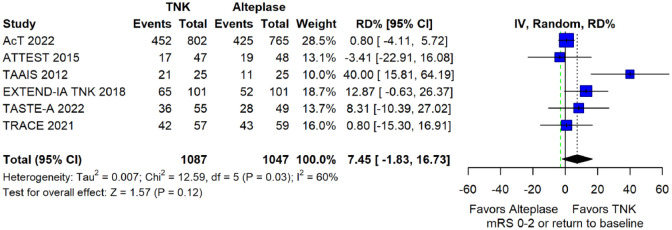
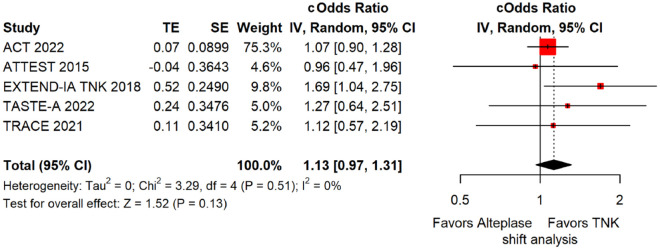
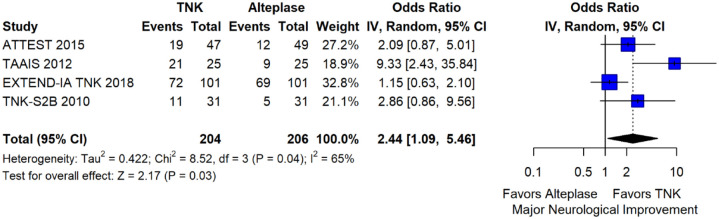
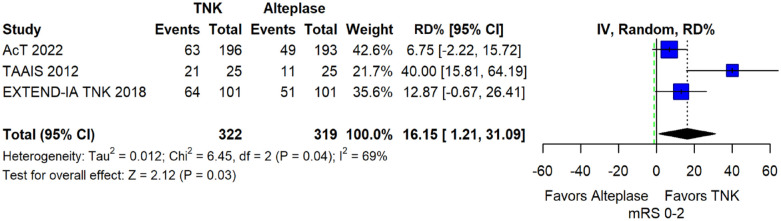
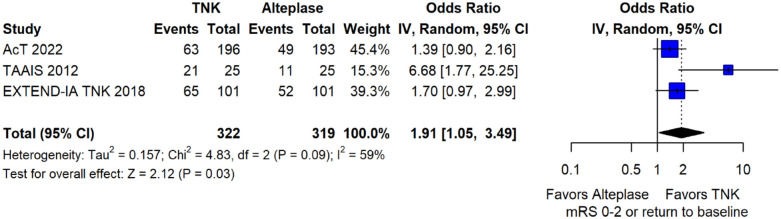
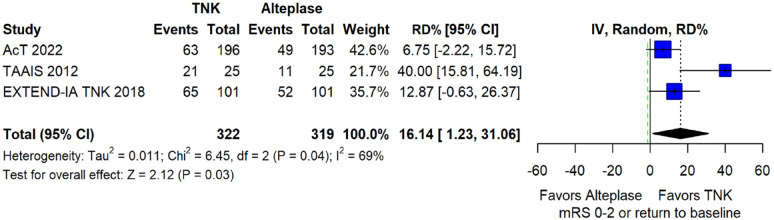
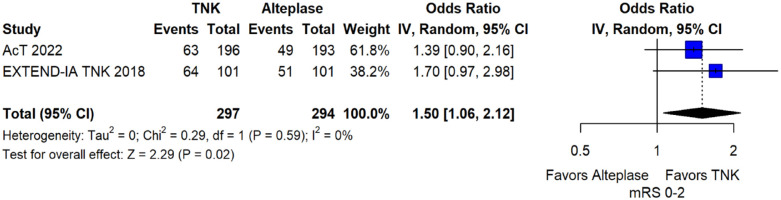
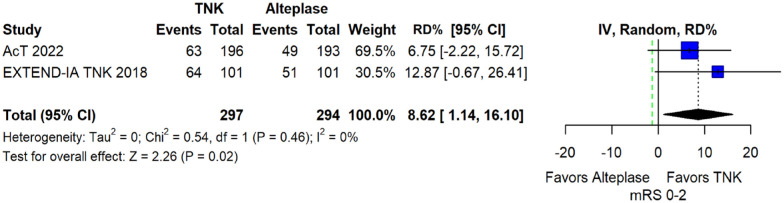
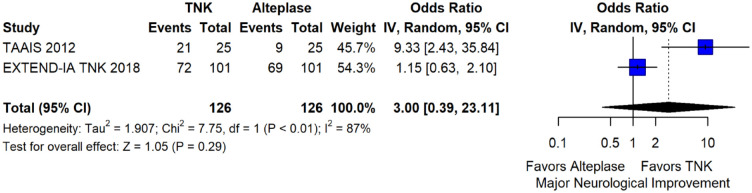
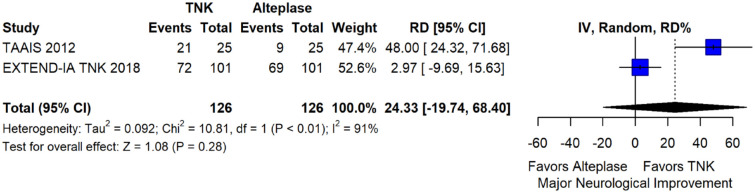
The pooled unadjusted OR for good functional outcome with tenecteplase was 1.36 (95%CI: 0.92–2.00; p = 0.12; I2 = 55%; Figure 8). The corresponding risk difference was 8.11% (95%CI: −1.41% to 17.62%; p = 0.10; I2 = 62%; Figure 9). Therefore, non-inferiority was met for good functional outcome based on our pre-specified 3% margin. Similar results were obtained when we conducted a sensitivity analysis for good functional outcome after additional inclusion of all patients returning to baseline mRS (Figures 10 and 11). The unadjusted common OR (cOR) for reduced disability with tenecteplase was 1.13 (95%CI: 0.97–1.31; p = 0.13; I2 = 0%; Figure 12). Tenecteplase was associated with higher odds of major neurological improvement, defined as a NIHSS reduction of at least 8 points at 24–72 h (OR = 2.44; 95%CI: 1.09–5.46; p = 0.03; I2 = 65%; Figure 13). We also conducted a sensitivity analysis after excluding EXTEND-IA TNK 17 that reported this outcome at 72 h, which also confirmed this association (Figure 14). With regard to the quality of life metrics, only the AcT trial 21 reported this outcome and found similar quality of life between tenecteplase and alteplase.
Figure 8.
Good functional outcome (mRS 0–2 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 9.
Pooled risk difference (in percent) for good functional outcome (mRS 0–2 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
Figure 10.
Sensitivity analysis for good functional outcome (mRS 0–2 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after additional inclusion of all patients returning to baseline mRS (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 11.
Sensitivity analysis for pooled risk difference (in percent) for good functional outcome (mRS 0–2 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after additional inclusion of all patients returning to baseline mRS (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
Figure 12.
Pooled unadjusted common odds ratio for reduced disability (improvement of a least 1 point on the mRS at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled cOR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; cOdds Ratio: common odds ratio; CI: confidence interval; TE: treatment effect; SE: standard error.
Figure 13.
Major neurological improvement at 24–72 h in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus with intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Figure 14.
Sensitivity analysis for major neurological improvement after excluding EXTEND-IA TNK4 that reported this outcome at 72 h in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
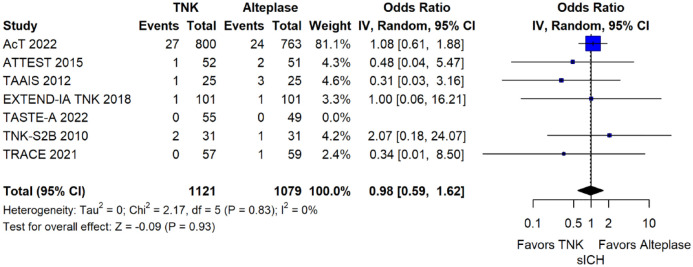
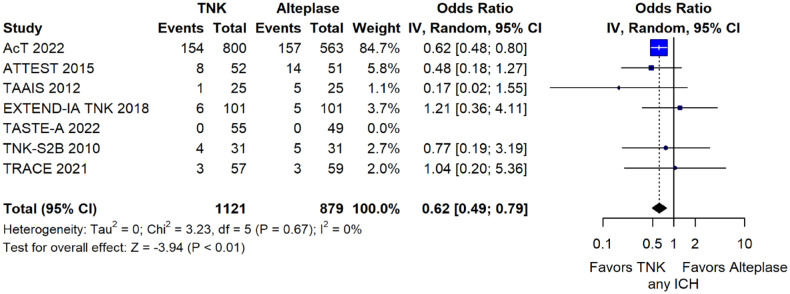
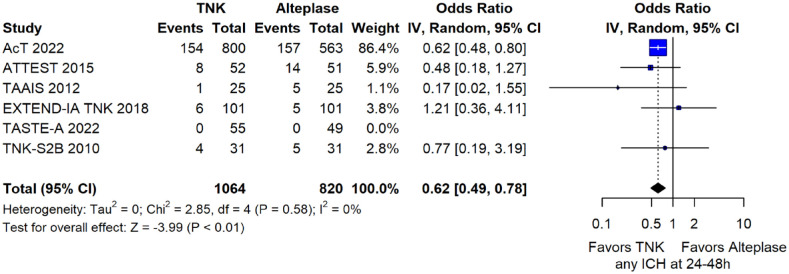
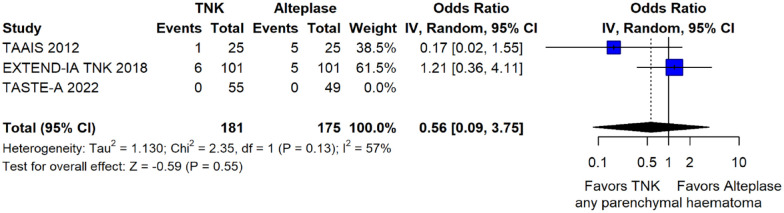
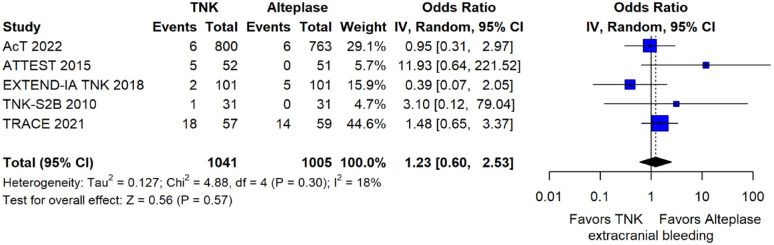
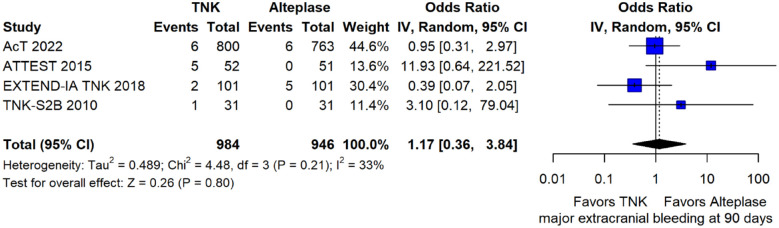
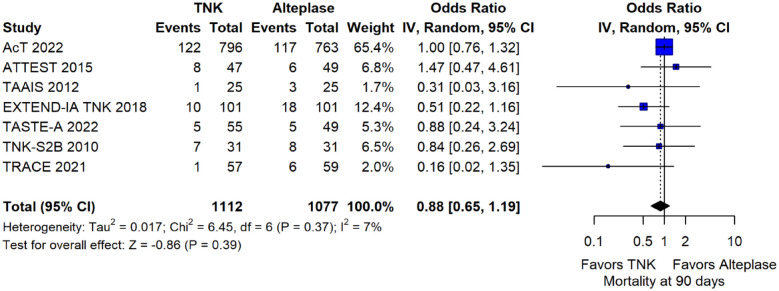
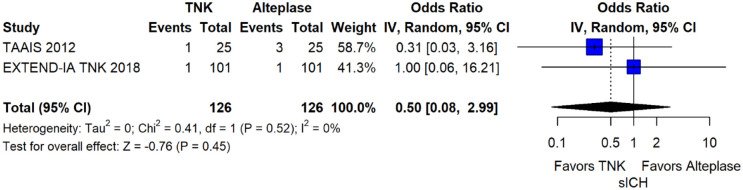
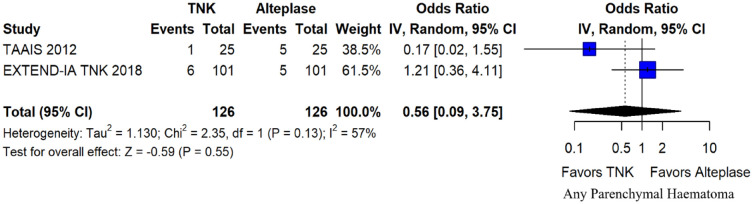
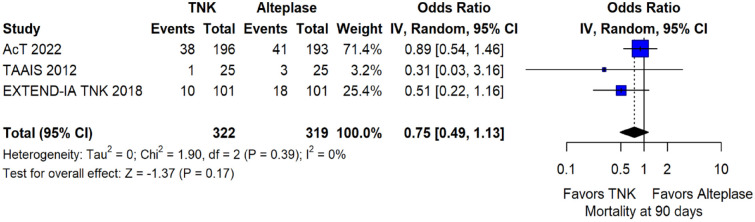
The rates of sICH according to individual study definition did not differ between treatment groups (OR = 0.98; 95%CI: 0.59–1.62; p = 0.93; I2 = 0%; Figure 15). A sensitivity analysis including the studies that reported sICH by the SITS-MOST definition (which was the most common available definition across all trials) yielded similar results (Figure 16). The rates of any intracranial haemorrhage were lower with tenecteplase compared to alteplase (OR = 0.62; 95%CI: 0.49–0.79; p < 0.01; I2 = 0%; Figure 17). We also conducted a sensitivity analysis after excluding TRACE 5 that reported this outcome at 90 days, which also confirmed this association (Figure 18). However, the rates of any parenchymal haematoma were not different across treatment groups (OR = 0.56; 95%CI: 0.09–3.75; p = 0.55; I2 = 57%; Figure 19). The rates of extracranial bleeding did not differ across treatment groups (OR = 1.23; 95%CI: 0.60–2.53; p = 0.57; I2 = 18%; Figure 20). A sensitivity analysis including the studies that reported major extracranial bleeding yielded similar results (Figure 21). All-cause mortality at 3 months was similar between the two treatment groups (OR = 0.88; 95%CI: 0.65–1.19; p = 0.39; I2 = 7%; Figure 22).
Figure 15.
Symptomatic intracranial haemorrhage according to individual study definition in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; sICH: Symptomatic intracranial haemorrhage.
Figure 16.
Sensitivity analysis for symptomatic intracranial haemorrhage according to SITS-MOST definition in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; sICH: Symptomatic intracranial haemorrhage.
Figure 17.
Any intracranial haemorrhage in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; ICH: intracranial haemorrhage.
Figure 18.
Sensitivity analysis for any intracranial haemorrhage in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after excluding TRACE5 that reported this outcome at 90 days (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; ICH: intracranial haemorrhage.
Figure 19.
Any parenchymal haematoma in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus with intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Figure 20.
Extracranial bleeding according to individual study reporting in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects metaanalysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Figure 21.
Sensitivity analysis for major extracranial bleeding in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Figure 22.
All-cause mortality at 3 months in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
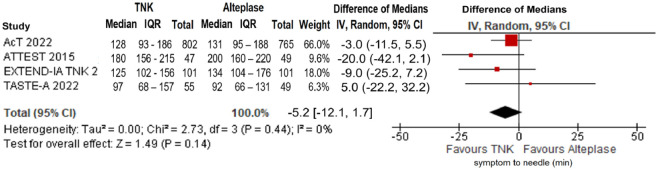
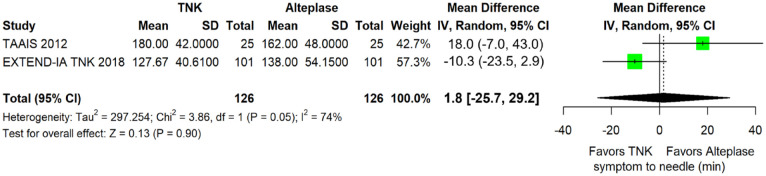
No significant difference in door-to-needle time was uncovered between the two treatment groups (difference in medians = −3.7 min; 95%CI: −9.5 to 2.2 min; p = 0.22; I2 = 83%; Figure 23). Similarly, there was no association of treatment with tenecteplase versus alteplase and elapsed time between symptom onset and bolus administration (difference in median onset-to-needle time = −5.2 min; 95%CI: −12.1 to 1.7 min; p = 0.14; I2 = 0%; Figure 24).
Figure 23.
Door-to-needle time (in minutes) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus with intravenous thrombolysis with alteplase 0.90 mg/kg (difference of medians, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; IQR: interquartile range.
Figure 24.
Symptom onset-to-needle time (in minutes) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (difference of medians, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; IQR: interquartile range.
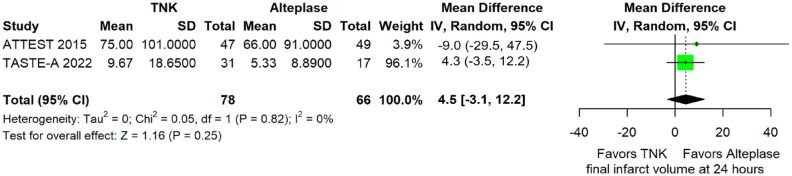
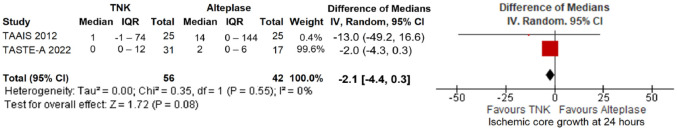
Final infarct volume did not differ between the two treatment arms (mean difference = 4.5 mL; 95%CI: −3.1 to 12.2 mL; p = 0.25; I2 = 0%; Figure 25). Furthermore, ischaemic core growth within the first 24 h was also similar across treatment groups (difference in medians = −2.1 mL; 95%CI: −4.4 to 0.3 mL; p = 0.08; I2 = 0%; Figure 26).
Figure 25.
Final infarct volume (in mL) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (mean difference, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; IQR: interquartile range.
Figure 26.
Ischaemic core growth (in mL) within the first 24 h in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (difference of medians, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; IQR: interquartile range.
Table 1 provides details regarding the assessment of the quality of evidence for all outcomes evaluated in PICO 1.1.
Table 1.
GRADE evidence profile for PICO 1.1.
| Certainty assessment |
No. of patients |
Effect |
Certainty |
Importance |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | TNK (0.25 mg/ kg) | Alteplase (0.9 mg/kg) | Relative (95% CI) | Absolute (95% CI) | ||
| Excellent functional outcome (modified Rankin Scale scores 0–1) at 90 days | ||||||||||||
| 7 | Randomised trials | Seriousa,b | Not serious | Not serious | Not serious | None | 449/1118 (40.2%) | 395/1079 (36.6%) | OR 1.17 (0.98 to 1.39) | 37 more per 1000 (from 5 fewer to 79 more) | ⊕ ⊕ ⊕ ○ Moderate | CRITICAL |
| Good functional outcome (modified Rankin Scale scores 0–2) at 90 days | ||||||||||||
| 6 | Randomised trials | Serious b | Serious c | Not serious | Not serious | None | 632/1087 (58.1%) | 575/1047 (54.9%) | OR 1.36 (0.92 to 2.00) | 74 more per 1000 (from 21 fewer to 160 more) | ⊕ ⊕ ○ ○ Low |
CRITICAL |
| Reduced disability (1 point or more reduction across all modified Rankin Scale scores) at 90 days | ||||||||||||
| 5 | Randomised trials | Serious b | Not serious | Not serious | Not serious | None | 1062 (N/A) | 1022 (N/A) | cOR 1.13 (0.97 to 1.31) | N/A | ⊕ ⊕ ⊕ ○Moderate | CRITICAL |
| Symptomatic intracranial haemorrhage (sICH) at 24–48 h | ||||||||||||
| 7 | Randomised trials | Seriousa,b | Not serious | Serious d | Not serious | None | 32/1121 (2.9%) | 32/1079 (3.0%) | OR 0.98 (0.59 to 1.62) | 1 fewer per 1000 (from 12 fewer to 18 more) | ⊕ ⊕ ○ ○ Low | CRITICAL |
| Mortality at 90 days | ||||||||||||
| 7 | Randomised trials | Seriousa,b | Not serious | Not serious | Not serious | None | 154/1112 (13.8%) | 163/1077 (15.1%) | OR 0.88 (0.65 to 1.19) | 17 fewer per 1,000 (from 49 fewer to 24 more) | ⊕ ⊕ ⊕ ○ Moderate |
CRITICAL |
| Major neurological improvement (according to definitions used in individual trials) at 24–72 h | ||||||||||||
| 4 | randomised trials | very seriousa,b | serious c | serious d | serious f | none | 123/204 (60.3%) | 95/206 (46.1%) | OR 2.44 (1.09 to 5.46) |
215 more per 1,000 (from 21 more to 363 more) |
⊕ ○ ○ ○ Very low |
IMPORTANT |
| Any intracranial haemorrhage (ICH) | ||||||||||||
| 7 | Randomised trials | Seriousa,b | Not serious | Serious d | Not serious | None | 176/1121 (15.7%) | 189/879 (21.5%) | OR 0.62 (0.49 to 0.79) | 73 fewer per 1000 (from 101 fewer to 41 fewer) | ⊕ ⊕ ○ ○ Low |
IMPORTANT |
| Extracranial bleeding | ||||||||||||
| 5 | Randomised trials | Seriousa,b | Not serious | Serious d | Not serious | None | 32/1041 (3.1%) | 25/1005 (2.5%) | OR 1.23 (0.60 to 2.53) | 6 more per 1000 (from 10 fewer to 36 more) |
⊕ ⊕ ○○ Low |
IMPORTANT |
| Final infarct volume at 24 h (cm 3 ) | ||||||||||||
| 2 | Randomised trials | Very seriousb,e | Not serious | Serious d | Not serious | None | 78 | 66 | N/A | 4.5 cm3 more (3.1 less to 12.2 more)* | ⊕ ○ ○ ○ Very low |
IMPORTANT |
| Ischaemic core growth within 24 h (cm 3 ) | ||||||||||||
| 2 | Randomised trials | Very serious e | Not serious | Not serious | Serious f | None | 56 | 42 | N/A | 2.1 cm3 less (4.4 less to 0.3 more)** | ⊕ ○ ○ ○ Very low |
IMPORTANT |
| Door-to-needle time (min) | ||||||||||||
| 2 | Randomised trials | Very serious e | Serious c | Not serious | Serious f | None | 856 | 805 | N/A | 3.7 min less (9.5 less to 2.2 more)** | ⊕ ○ ○ ○ Very low |
IMPORTANT |
| Onset-to-treatment time (min) | ||||||||||||
| 4 | Randomised trials | Serious b | Not serious | Not serious | Serious f | None | 1087 | 1048 | N/A | 5.2 min less (12.1 less to 1.7 more)** | ⊕ ⊕ ○ ○ Low | IMPORTANT |
TNK: tenecteplase, CI: confidence interval; MD: mean difference; OR: odds ratio; cOR: common odds ratio; N/A: not applicable.
Based on differences in pooled means (since there was no available data on medians with corresponding interquartile ranges in ⩾2 studies).
Based on differences in pooled medians.
Concerns from premature termination of one study (TNK-S2B 2010).
Concerns due to lack of blinding in outcomes assessment in one study (ATTEST).
Presence of heterogeneity.
Use of different outcome definitions across studies.
Evidence derived from less than half of the total studies population.
Wide and/or inconclusive confidence intervals.

Additional information
A previous meta-analysis including data from 5 RCTs has provided preliminary data favouring non-inferiority of tenecteplase compared to alteplase using three non-inferiority margins for 3-month excellent functional outcome: 6.5% (lead non-inferiority margin), 5.0% and 1.3% (more stringent non-inferiority margins). 37 Nevertheless, there are methodological concerns with regard to the aforementioned non-inferiority meta-analysis 37 since different tenecteplase dose tiers were pooled together, severe strokes were under-represented and potentially arbitrary statistical assumptions were employed. In particular, an assumption was made to split the alteplase control group into two half-sized groups for tenecteplase-alteplase comparisons across the two tenecteplase dose tiers in TAAIS 16 trial. Finally, the TNK-S2B 13 trial reported pooled rates of outcome across the alteplase groups and did not provide detailed information regarding outcomes in patients randomised to the alteplase arm across the three tenecteplase dose tiers. Thus, it is unclear how the authors of the non-inferiority meta-analysis 37 were able to compare different tenecteplase doses with the respective alteplase arm across the three tiers of the RCTs.
Although our meta-analysis of RCTs did not suggest a significant reduction in time metrics, there is mounting observational data reporting an improved service delivery in hospitals and health services without safety concerns with the off-label use of tenecteplase in everyday clinical practice across hospital settings.12,38–45 Furthermore, a recent meta-analysis of observational studies has documented similar safety and improved effectiveness in AIS patients receiving off-label IVT with tenecteplase compared to standard dose alteplase. 46

1.2 For patients with acute ischaemic stroke of <4.5 h duration, does intravenous thrombolysis with tenecteplase 0.40 mg/kg compared with intravenous thrombolysis with alteplase 0.90 mg/kg lead to:
(a) a non-inferior proportion of patients with excellent functional outcome (mRS scores of 0–1) at 90 days?
(b) non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, good functional outcome defined by mRS 0–2 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)?
(c) a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)?
(d) a reduction in key time metrics (onset-to-treatment time, door-to-needle time)?
(e) an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth within the first 24 h)?
Analysis of current evidence
The literature search identified three published RCTs addressing this PICO question. TNK-S2B 13 has already been discussed in PICO 1.1
NOR-TEST 14 (the Norwegian Tenecteplase Stroke Trial) was a phase 3 RCT with PROBE design that randomised 1100 AIS patients in Norway admitted within 4.5 h of symptom onset or within 4.5 h of awakening with symptoms, or who were eligible for bridging therapy before MT in two treatment arms: tenecteplase 0.40 mg/kg or alteplase 0.9 mg/kg. The primary outcome of interest was the rate of excellent functional outcome at 3 months. Excellent functional outcome was achieved by 64% patients in the tenecteplase group and 63% patients in the alteplase group (odds ratio 1.08, 95% CI 0.84–1.38; p = 0.52), while safety profile was similar between the two treatment groups. It should be underscored, however, that the majority of the patients enrolled in this study had mild strokes (median NIHSS score of 4 points), while 17% of randomised patients were stroke mimics.
NOR-TEST 2 part A (the Norwegian Tenecteplase Stroke Trial 2) was a non-inferiority phase 3 RCT with PROBE design that randomised 204 AIS patients with moderate or severe strokes (defined as NIHSS-score at admission of ⩾6) admitted within 4.5 h of symptom onset in Norway in two treatment arms: tenecteplase 0.40 mg/kg or alteplase 0.9 mg/kg. 22 The primary outcome of interest was the rates of excellent functional outcome at 3 months. However, the trial was prematurely terminated due to safety reasons. Excellent functional outcome was less likely in the tenecteplase group (unadjusted OR 0.45; 95%CI: 0.25–0.80; p = 0.0064). Both any intracranial haemorrhage (unadjusted OR 3.68; 95%CI: 1.49–9.11; p = 0.0031) and mortality (unadjusted OR 3.56; 95%CI: 1.24–10.21; p = 0.013) were more frequent in the tenecteplase group, while there were numerically more cases of sICH with tenecteplase (6%) than with alteplase (1%; p = 0.061).
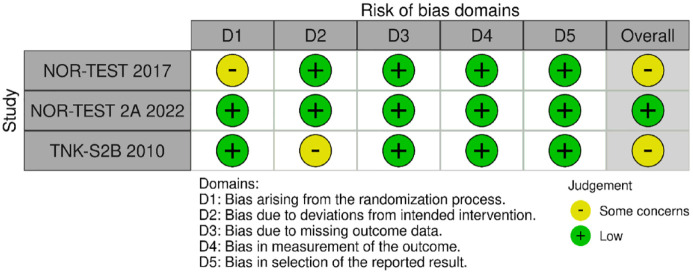
MWG assessment of the risk of bias in each RCT according to the Cochrane RoB-2 tool with regard to excellent functional outcome at 90 days is presented in Figure 27. NOR-TEST 2 22 was considered to be at overall low risk of bias, while NOR-TEST 14 and TNK-S2B 13 presented some concerns during the randomisation process and due to small deviations from intended interventions, respectively.
Figure 27.
Risk of bias in each randomised-controlled clinical trial of IVT with tenecteplase at a dose of 0.4 mg/kg versus IVT with alteplase 0.9 mg/kg for AIS patients, with regards to excellent functional outcome at 90 days.
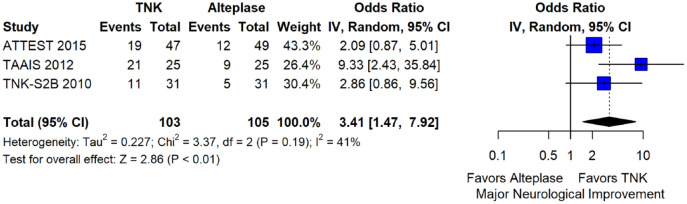
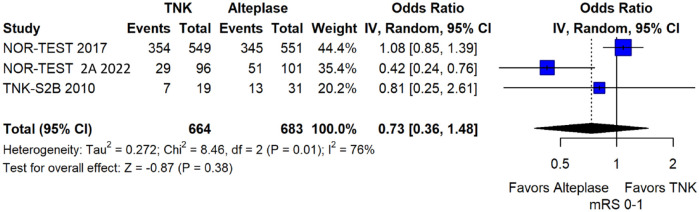
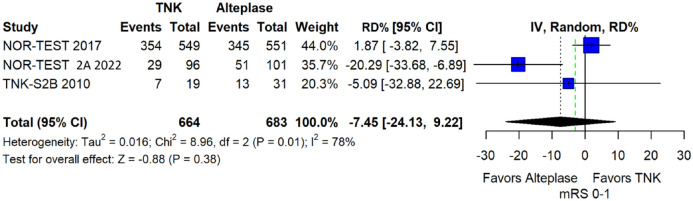
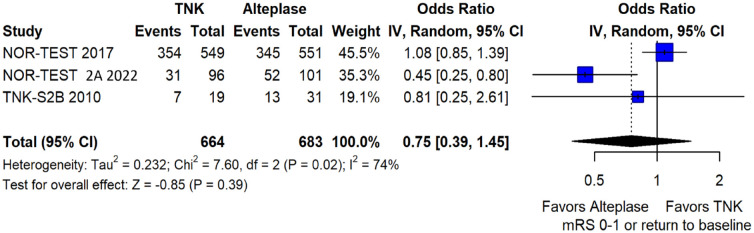
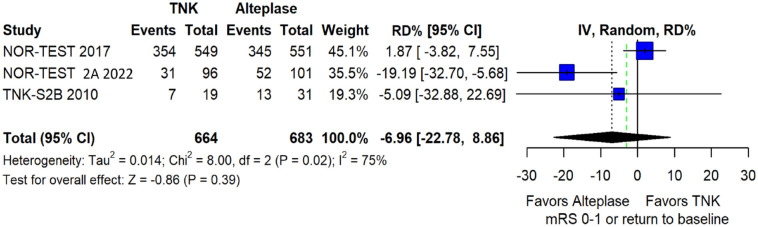
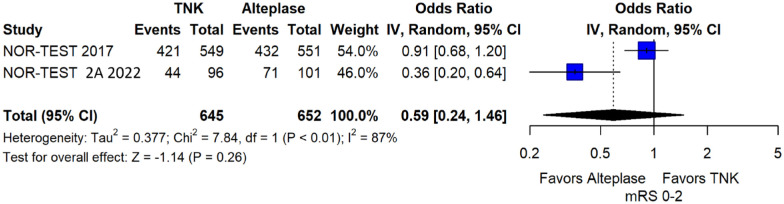
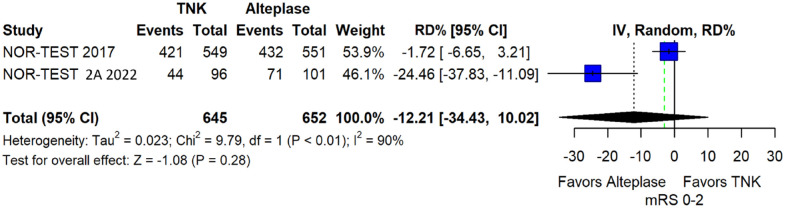
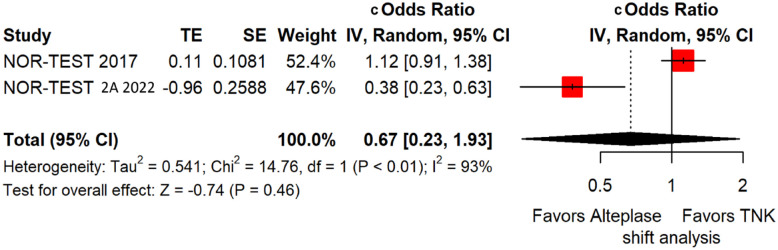
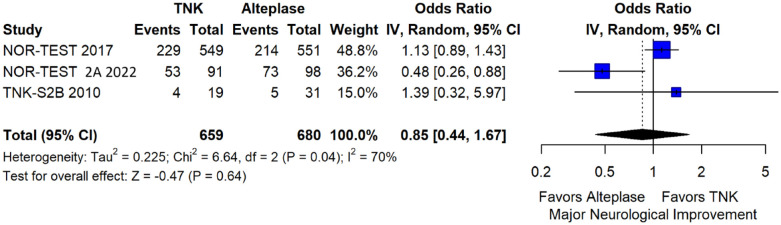
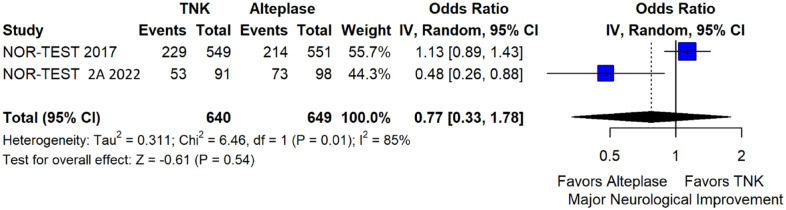
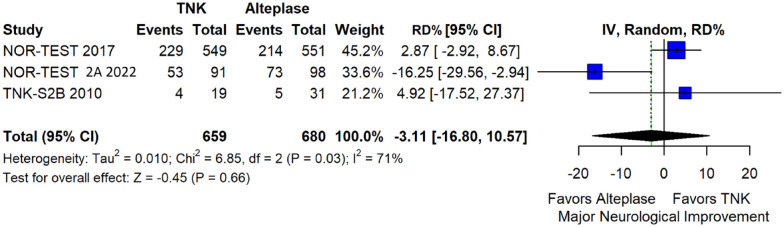
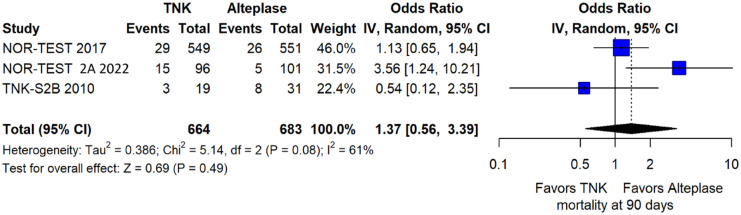
We conducted a study-level random-effects meta-analysis of the three RCTs comparing IVT with 0.40 mg/kg tenecteplase versus IVT with 0.9 mg/kg alteplase, comprising a total of 1347 AIS patients. Compared to patients randomised to IVT with alteplase, the pooled unadjusted OR for excellent functional outcome in patients randomised to IVT with tenecteplase 0.40 mg/kg was 0.73 (95%CI: 0.36–1.48; p = 0.38; I2 = 76%; Figure 28). The corresponding risk difference was −7.45% (95%CI: −24.13% to 9.22%; p = 0.38; I2 = 78%; Figure 29). Therefore, non-inferiority was not met for excellent functional outcome (mRS 0–1) based on our pre-specified 3% margin. Similar results were obtained when we conducted a sensitivity analysis for excellent functional outcome after additional inclusion of all patients returning to baseline mRS (Figures 30 and 31). The pooled unadjusted OR for good functional outcome with tenecteplase was 0.59 (95%CI: 0.24–1.46; p = 0.26; I2 = 87%; Figure 32). The corresponding risk difference was −12.21% (95%CI: −34.43% to 10.02%; p = 0.28; I2 = 90%; Figure 33). Therefore, non-inferiority was not met for good functional outcome based on our pre-specified 3% margin. The unadjusted cOR for reduced disability with tenecteplase compared to alteplase was 0.67 (95%CI: 0.23–1.93; p = 0.46; I2 = 93%; Figure 34). Similar odds of major neurological improvement at 24 h were observed between the two treatment arms (OR = 0.85; 95%CI: 0.44–1.67; p = 0.64; I2 = 70%; Figure 35). A sensitivity analysis, after excluding TNK-S2B 13 that defined major neurological improvement as a NIHSS reduction of at least 8 points (in contrast to NOR-TEST 14 and NORT-TEST 2 22 that defined as major neurological improvement a NIHSS reduction of at least 4 points), yielded similar results (Figure 36). The risk difference for major neurological improvement was −3.11% (95%CI: −16.80% to 10.57%; p = 0.66; I2 = 71%; Figure 37).
Figure 28.
Excellent functional outcome (mRS 0–1 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 29.
Pooled risk difference (in percent) for excellent functional outcome (mRS 0–1 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
Figure 30.
Sensitivity analysis for excellent functional outcome (mRS 0–1 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after additional inclusion of all patients returning to baseline mRS (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 31.
Sensitivity analysis for pooled risk difference (in percent) for excellent functional outcome (mRS 0–1 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus with intravenous thrombolysis with alteplase 0.90 mg/kg, after additional inclusion of all patients returning to baseline mRS (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
Figure 32.
Good functional outcome (mRS 0–2 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 33.
Pooled risk difference (in percent) for good functional outcome (mRS 0–2 at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
Figure 34.
Pooled unadjusted common odds ratio for reduced disability (improvement of at least 1 point on the mRS at 90 days) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled cOR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; cOdds Ratio: common odds ratio; CI: confidence interval; TE: treatment effect; SE: standard error.
Figure 35.
Major neurological improvement within 24 h in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Figure 36.
Sensitivity analysis for major neurological improvement within 24 h, after excluding TNK-S2B 1 that defined major neurological improvement as a NIHSS reduction of at least 8 (in contrast to NOR-TEST 8 and NORT-TEST 2 9 that accounted as major neurological improvement a NIHSS reduction of at least 4), in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Figure 37.
Pooled risk difference (in percent) for major neurological improvement within 24 h in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
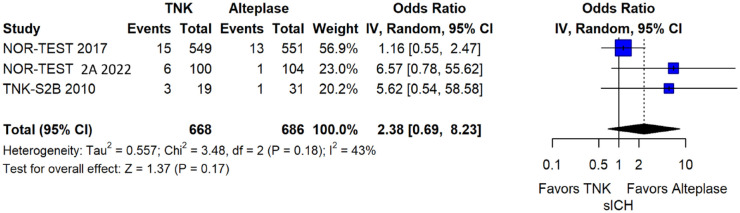
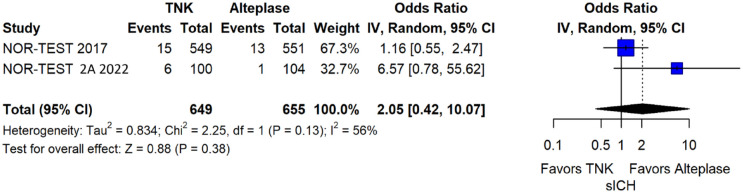
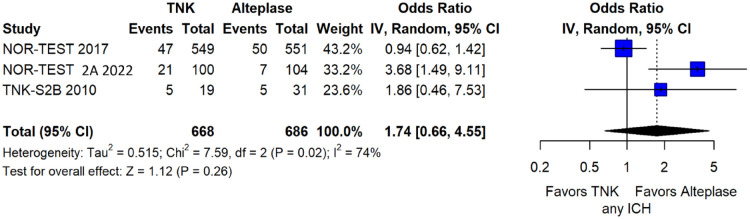
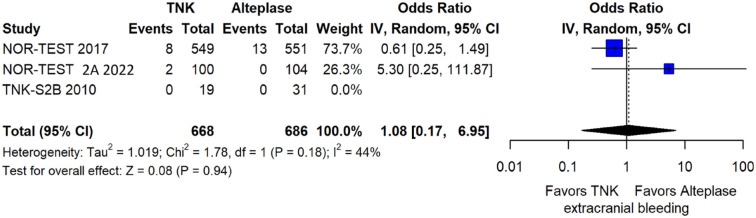
The rates of sICH according to individual study definition did not significantly differ across treatment groups (OR = 2.38; 95%CI: 0.69–8.23; p = 0.17; I2 = 43%; Figure 38). A sensitivity analysis including the studies that reported sICH by the ECASS III definition (which was the most common available definition across all trials) yielded similar results (Figure 39). The rates of any intracranial haemorrhage did not significantly differ with tenecteplase compared to alteplase (OR = 1.74; 95%CI: 0.66–4.55; p = 0.26; I2 = 74%; Figure 40). The rates of extracranial bleeding did not differ across treatment arms (OR = 1.08; 95%CI: 0.17–6.95; p = 0.94; I2 = 44%; Figure 41). All-cause mortality at 3 months was similar between the two treatment groups (OR = 1.37; 95%CI: 0.56–3.39; p = 0.49; I2 = 61%; Figure 42).
Figure 38.
Symptomatic intracranial haemorrhage according to individual study definition in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; sICH: symptomatic intracranial haemorrhage.
Figure 39.
Sensitivity analysis for symptomatic intracranial haemorrhage according to ECASS III definition in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; sICH: Symptomatic intracranial haemorrhage.
Figure 40.
Any intracranial haemorrhage in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; ICH: intracranial haemorrhage.
Figure 41.
Extracranial bleeding in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Figure 42.
All-cause mortality at 3 months in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
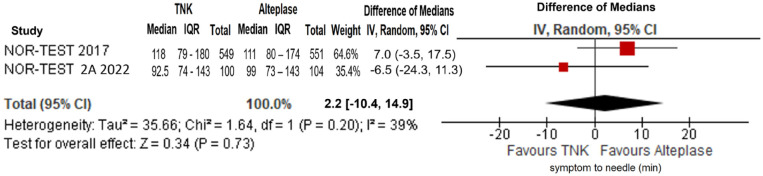
No difference in onset-to-needle time was uncovered between the two treatment arms (difference in medians= 2.2 min; 95%CI: −10.4 to 14.9 min; p = 0.73; I2 = 39%; Figure 43).
Figure 43.
Symptom onset-to-needle time (in minutes) in patients with acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.40 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (difference of medians, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; IQR: interquartile range.
Table 2 provides details regarding the assessment of the quality of evidence for all outcomes assessed in PICO 1.2.
Table 2.
GRADE evidence profile for PICO 1.2.
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | TNK (0.4 mg/kg) | Alteplase (0.9 mg/kg) | Relative (95% CI) | Absolute (95% CI) | ||
| Excellent functional outcome (modified Rankin Scale scores 0–1) at 90 days | ||||||||||||
| 3 | Randomised trials | Serious a | Serious b | Not serious | Serious c | None | 390/664 (58.7%) | 409/683 (59.9%) | OR 0.73 (0.36 to 1.48) | 77 fewer per 1000 (from 249 fewer to 90 more) | ⊕⊕○○ Low | CRITICAL |
| Good functional outcome (modified Rankin Scale scores 0–2) at 90 days | ||||||||||||
| 2 | Randomised trials | Serious d | Serious b | Not serious | Serious c | None | 465/645 (72.1%) | 503/652 (77.1%) | OR 0.59 (0.24 to 1.46) | 106 fewer per 1000 (from 324 fewer to 60 more) | ⊕⊕○○Low | CRITICAL |
| Reduced disability (1 point or more reduction across all modified Rankin Scale scores) at 90 days | ||||||||||||
| 2 | Randomised trials | Serious d | Serious b | Not serious | Serious c | None | 645 (N/A) | 652 (N/A) | cOR 0.67 (0.23 to 1.93) | N/A | ⊕⊕○○Low | CRITICAL |
| Symptomatic intracranial haemorrhage (sICH) at 24–48 h | ||||||||||||
| 3 | Randomised trials | Serious a | Not serious | Serious e | Serious c | None | 24/668 (3.6%) | 15/686 (2.2%) | OR 2.38 (0.69 to 8.23) | 29 more per 1000 (from 7 fewer to 136 more) | ⊕○○○Very low | CRITICAL |
| Mortality at 90 days | ||||||||||||
| 3 | Randomised trials | Serious a | Serious b | Not serious | Serious c | None | 47/664 (7.1%) | 39/683 (5.7%) | OR 1.37 (0.56 to 3.39) | 20 more per 1000 (from 25 fewer to 113 more) | ⊕○○○Very low | CRITICAL |
| Major neurological improvement (according to definitions used in individual trials) at 24–72 h | ||||||||||||
| 3 | Randomised trials | Serious a | Serious b | Serious e | Serious c | None | 286/659 (43.4%) | 292/680 (42.9%) | OR 0.85 (0.44 to 1.67) | 39 fewer per 1000 (from 181 fewer to 127 more) | ⊕○○○Very low | IMPORTANT |
| Any intracranial haemorrhage (ICH) | ||||||||||||
| 3 | Randomised trials | Serious a | Serious b | Not serious | Serious c | None | 73/668 (10.9%) | 62/686 (9.0%) | OR 1.74 (0.66 to 4.55) | 57 more per 1000 (from 29 fewer to 221 more) | ⊕○○○ Very low |
IMPORTANT |
| Onset-to-treatment time (min) | ||||||||||||
| 2 | Randomised trials | Serious d | Not serious | Not serious | Serious c | None | 649 | 655 | N/A | 2.2 min more (10.4 less to 14.9 more)* | ⊕⊕○○Low | IMPORTANT |
| Extracranial bleeding | ||||||||||||
| 3 | Randomised trials | Serious a | Not serious | Serious e | Serious c | None | 10/668 (1.5%) | 13/686 (1.9%) | OR 1.08 (0.17 to 6.95) |
2 more per 1000 (from 16 fewer to 103 more) | ⊕○○○ Very low |
IMPORTANT |
TNK: tenecteplase; CI: confidence interval; MD: mean difference; OR: odds ratio; cOR: common odds ratio; N/A: not applicable.
Based on differences in pooled medians.
Concerns from premature termination of one study (TNK-S2B 2010).
Presence of heterogeneity.
Wide and/or inconclusive confidence intervals.
Included studies are from the same research group.
Use of different outcome definitions across studies.

Additional information
In an open-label, dose-escalation safety study that was conducted to develop initial experience with tenecteplase in AIS, treatment investigators report 0% and 32% rates for sICH and asymptomatic intracranial haemorrhage, respectively, among the 25 patients treated with tenecteplase 0.4 mg/kg. 9 EXTEND-IA TNK part 2 was a phase 2 RCT with PROBE design that randomised 300 LVO patients who were eligible to undergo MT within 4.5 h from symptom onset in Australia and New Zealand in two treatment arms: tenecteplase 0.25 mg/kg and tenecteplase 0.40 mg/kg. 18 The primary endpoint was reperfusion of greater than 50% of the involved ischaemic territory or an absence of retrievable thrombus at the time of the initial angiographic assessment. The number of participants with greater than 50% reperfusion of the previously occluded vascular territory was 29/150 (19.3%) in the 0.40 mg/kg group versus 29/150 (19.3%) in the 0.25 mg/kg group (adjusted risk ratio, 1.03; 95%CI: 0.66–1.61; p = 0.89). Among the 6 secondary outcomes, there were no significant differences in any of the functional outcomes between the 0.40 mg/kg and 0.25 mg/kg groups nor in all-cause deaths (17% vs 15%) or sICH (4.7% vs 1.3%; unadjusted risk difference, 3.3%; 95% CI: −0.5% to 7.2%). Although the difference was not statistically significant, the numerically higher rates of sICH may indicate a potential higher sICH risk in patients treated with tenecteplase 0.40 mg/kg compared those treated with tenecteplase 0.25 mg/kg.
In a network meta-analysis including data from 5 RCTs with a total of 1585 patients, similar safety (mortality, intracranial haemorrhage, sICH) and efficacy outcomes (excellent functional outcome, good functional outcome, complete or partial recanalisation) were found for tenecteplase dose of 0.40 mg/kg and alteplase dose of 0.90 mg/kg. 47 Yet, the results of this study-level network meta-analysis should be considered with caution, since they are derived by indirect comparisons that may also explain the perceived inconsistencies compared to the results of NOR-TEST 2 part A. 22
1.3 In patients with acute ischaemic stroke of <4.5 h duration with prehospital management with a mobile stroke unit does intravenous thrombolysis with tenecteplase 0.25 mg/kg compared with intravenous thrombolysis with alteplase 0.90 mg/kg lead to:
(a) a non-inferior proportion of patients with excellent functional outcome (mRS scores of 0–1) at 90 days?
(b) non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, good functional outcome defined by mRS 0–2 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)?
(c) a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)?
(d) a reduction in key time metrics (onset-to-treatment time, door-to-needle time)?
e) an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth within the first 24 h)?
Analysis of current evidence
The literature search identified one RCT addressing this PICO question.
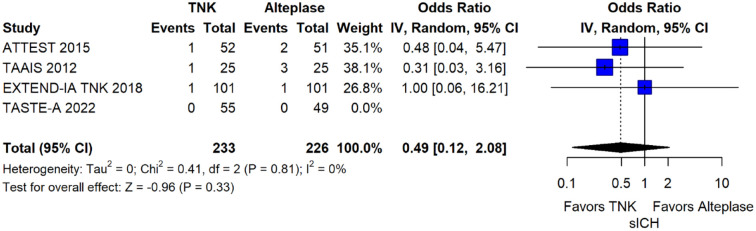
This RCT was the TASTE-A 20 trial, which has been already discussed in PICO 1.1. In brief, TASTE-A provided evidence that ultra-early tenecteplase at a dose of 0.25 mg/kg compared to standard-dose alteplase, both given in MSUs, reduced the volume of the post-treatment perfusion lesion, led to greater ultra-early clinical recovery [greater reduction in the pre-specified secondary efficacy outcome of median NIHSS between MSU and emergency department arrival when treated with tenecteplase (median NIHSS reduction 1, IQR 0–6)], and was initiated faster than alteplase on the MSU in AIS patients. More specifically, patients treated with tenecteplase had a significantly shorter time from MSU CT imaging to initiation of thrombolytic treatment (median 13 min, IQR 9–18) compared with patients treated with alteplase (median 19 min, 14–27; adjusted difference in medians –6.1, 95%CI –9.6 to –2.6; p = 0.0010). No significant differences were observed between patients treated with tenecteplase compared to alteplase on early neurological deterioration, NIHSS-scores at 24 h, functional outcomes at 3 months and death at 3 months. The rates of sICH and other bleeding events were similar between the two treatment groups.
Table 3 provides details regarding the assessment of the quality of evidence for all outcomes evaluated in PICO 1.3.
Table 3.
GRADE evidence profile for PICO 1.3.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | TNK (0.25 mg/ kg) | Alteplase (0.9 mg/kg) | Relative (95% CI) | Absolute (95% CI) | ||
| Excellent functional outcome (modified Rankin Scale scores 0–1) at 90 days | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Serious a | Evidence provided by a single phase 2 trial | 23/55 (41.8%) | 20/49 (40.8%) | OR 1.04 (0.48 to 2.28) | 10 more per 1000 (from 159 fewer to 203 more) | ⊕⊕○○Low | CRITICAL |
| Good functional outcome (modified Rankin Scale scores 0–2) at 90 days | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Serious a | Evidence provided by a single phase 2 trial | 36/55 (65.5%) | 26/49 (53.1%) | OR 1.68 (0.76 to 3.69) | 124 more per 1000 (from 68 fewer to 276 more) | ⊕⊕○○Low | CRITICAL |
| Reduced disability (1 point or more reduction across all modified Rankin Scale scores) at 90 days | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Serious a | Evidence provided by a single phase 2 trial | 55 (N/A) | 49 (N/A) | cOR 1.03 (0.51 to 2.08) | 1 fewer per 1000 (from 2 fewer to 1 fewer) | ⊕⊕○○Low | CRITICAL |
| Symptomatic intracranial haemorrhage (sICH) within 36 h | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Very serious b | Evidence provided by a single phase 2 trial | 0/55 (0.0%) | 0/49 (0.0%) | Not estimable | Not estimable | ⊕○○○Very low | CRITICAL |
| Mortality at 90 days | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Serious a | Evidence provided by a single phase 2 trial | 5/55 (9.1%) | 5/49 (10.2%) | OR 0.88 (0.24 to 3.24) | 11 fewer per 1000 (from 75 fewer to 167 more) | ⊕⊕○○Low | CRITICAL |
| Any intraparenchymal haematoma | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Very serious b | Evidence provided by a single phase 2 trial | 0/55 (0.0%) | 0/49 (0.0%) | Not estimable | Not estimable | ⊕○○○Very low | IMPORTANT |
| Final infarct volume at 24 h (cm 3 ) | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Serious a | Evidence provided by a single phase 2 trial | 31 | 17 | N/A | MD 4.3 cm3 more (3.5 less to 12.1 more) | ⊕⊕○○Low | IMPORTANT |
| Ischaemic core growth within 24 h (cm 3 ) | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Serious a | Evidence provided by a single phase 2 trial | 31 | 17 | N/A | MD 1.3 cm3 more (2.7 less to 5.3 more) | ⊕⊕○○Low | IMPORTANT |
| MSU arrival to needle time (min) | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Serious a | Evidence provided by a single phase 2 trial | 54 | 49 | N/A | MD 6.3 min less (9.8 less to 2.8 more) | ⊕⊕○○Low | IMPORTANT |
| Onset to treatment time (min) | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Serious a | Evidence provided by a single phase 2 trial | 55 | 49 | N/A | MD 11 min more (11.7 less to 33.7 more) | ⊕⊕○○Low | IMPORTANT |
| Reperfusion at 24 h | ||||||||||||
| 1 | Randomised trial | Not serious | N/A | Not serious | Serious a | Evidence provided by a single phase 2 trial | 35/55 (63.6%) | 35/49 (71.4%) | OR 0.70 (0.31 to 1.60) | 78 fewer per 1000 (from 278 fewer to 86 more) | ⊕⊕○○Low | IMPORTANT |
TNK: tenecteplase, CI: confidence interval; MD: mean difference; OR: odds ratio; cOR: common odds ratio; N/A: not applicable.
Wide confidence intervals.
No events of interest.

Additional information
We were unable to identify any observational studies evaluating the safety and effectiveness of tenecteplase 0.25 mg/kg compared to alteplase in the prehospital setting. However, it should be noted that a recent study reported that of 497 AIS patients treated with alteplase on a single-centre MSU, 41 (8.3%) had delay or interruption of the infusion for reasons that did not reflect either a side effect or contraindication to alteplase. 48 This observation provides an opportunity for more complete and faster treatment with tenecteplase compared to alteplase in the prehospital settings.
PICO 2 Patients <4.5 h and large vessel occlusion
For large vessel occlusion acute ischaemic stroke patients of <4.5 h duration does intravenous thrombolysis with tenecteplase 0.25 mg/kg compared with intravenous thrombolysis with alteplase 0.90 mg/kg lead to:
a) a non-inferior proportion of patients with good functional outcome (mRS scores of 0–2) at 90 days?
b) non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, excellent functional outcome defined by mRS 0–1 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)?
c) a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)?
d) a reduction in key time metrics (onset-to-treatment time, door-to-needle time)?
e) an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth at 24 h, recanalisation at 24 h, recanalisation at the end of mechanical thrombectomy, recanalisation before mechanical thrombectomy at first angiographic acquisition or averted mechanical thrombectomy)?
Analysis of current evidence
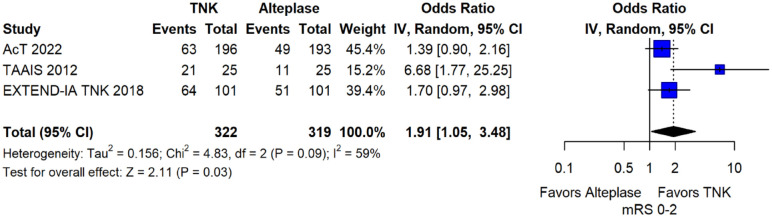
The literature search identified four published RCTs addressing this PICO question.
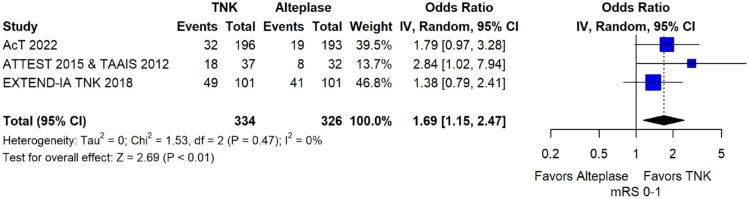
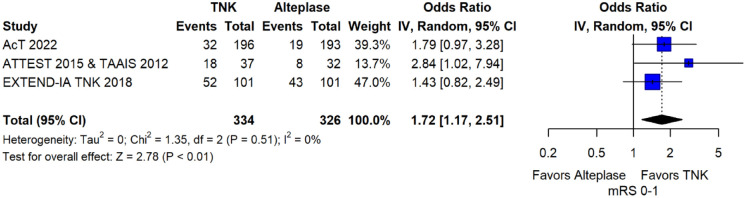
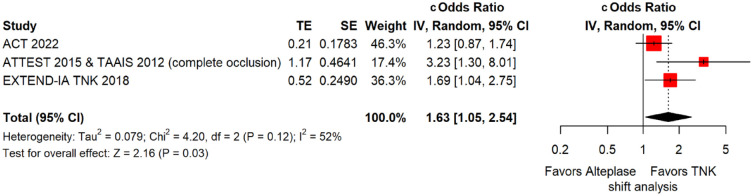
These RCTs are TAAIS, 16 ATTEST, 15 EXTEND-IA TNK 17 and AcT 21 and have already been discussed in PICO 1.1. Importantly, for TAAIS 16 and ATTEST 15 several outcomes have been presented in an individual patient data analysis conducted by Bivard et al., 49 which allowed the central assessment of occlusion status at baseline and at 24 h post thrombolysis. This study showed that patients with complete occlusion experienced more frequently early clinical improvement [median NIHSS reduction at 24 h : 9 (IQR = 6) vs 1 (IQR = 1); p = 0.001] and had higher rates of excellent functional outcome at 3 months (OR: 4.82; 95% CI: 1.02–7.84; p = 0.05) when treated with tenecteplase compared to alteplase.
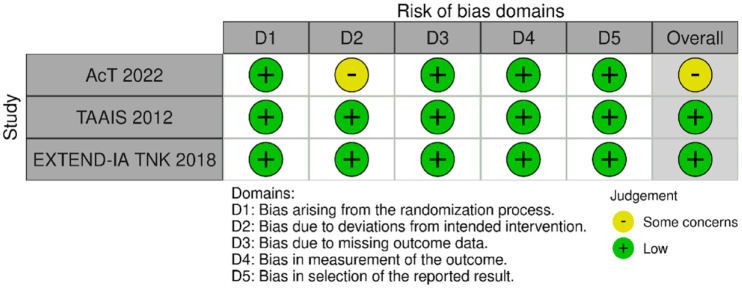
MWG assessment of the risk of bias in each RCT according to the Cochrane RoB-2 tool with regards to good functional outcome at 90 days is presented in Figure 44. All studies were considered to be at overall low risk of bias except for AcT, 21 which presented some concerns due to small deviations from intended interventions.
Figure 44.
Risk of bias in each randomised-controlled clinical trials-controlled clinical trial of IVT with tenecteplase at a dose of 0.25 mg/kg versus IVT with alteplase for AIS patients with large vessel occlusion, with regard to good functional outcome at 90 days.
We conducted a study-level random-effects meta-analysis of the four RCTs comparing IVT with 0.25 mg/kg tenecteplase versus IVT with 0.9 mg/kg alteplase, comprising a total of 660 AIS patients with large vessel occlusion. Compared to patients randomised to IVT with alteplase the pooled unadjusted OR for good functional outcome in patients randomised to IVT with tenecteplase was 1.91 (95%CI: 1.05–3.48; p = 0.03; I2 = 59%; Figure 45). The corresponding risk difference was 16.15% (95%CI: 1.21% to 31.09%; p = 0.03; I2 = 69%; Figure 46). Therefore, both non-inferiority and superiority were met for good functional outcome. Similar results were obtained when we conducted a sensitivity analysis for good functional outcome after additional inclusion of all patients returning to baseline mRS (Figures 47 and 48). An additional sensitivity analysis was conducted after excluding TAAIS 16 that enrolled patients with both LVO and more distal occlusions. This sensitivity analysis yielded similar results (Figures 49 and 50) to the primary analysis. The pooled unadjusted OR for excellent functional outcome with tenecteplase was 1.69 (95%CI: 1.15–2.47; p < 0.01; I2 = 0%; Figure 51). Similar results were obtained when we conducted a sensitivity analysis for excellent functional outcome after additional inclusion of all patients returning to baseline mRS (Figure 52). The unadjusted cOR for reduced disability with tenecteplase was 1.63 (95%CI: 1.05–2.54; p = 0.03; I2 = 52%; Figure 53). Major neurological improvement at 24–72 h did not significantly differ between the two treatment arms (OR = 3.00; 95%CI: 0.39–23.11; p = 0.29; I2 = 87%; Figure 54). The corresponding risk difference was 24.33% (95%CI: −19.74% to 68.40%; p = 0.28; I2 = 91%; Figure 55).
Figure 45.
Good functional outcome (mRS 0–2 at 90 days) in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 46.
Pooled risk difference (in percent) for good functional outcome (mRS 0–2 at 90 days) in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −1.3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
Figure 47.
Sensitivity analysis for good functional outcome (mRS 0–2 at 90 days) in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after additional inclusion of all patients returning to baseline mRS (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 48.
Sensitivity analysis for pooled risk difference (in percent) for good functional outcome (mRS 0–2 at 90 days) in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after additional inclusion of all patients returning to baseline mRS (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −1.3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
Figure 49.
Sensitivity analysis for good functional outcome (mRS 0–2 at 90 days) in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after excluding TAAIS 2 (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 50.
Sensitivity analysis for pooled risk difference (in percent) for good functional outcome (mRS 0–2 at 90 days) in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg after excluding TAAIS 2 (unadjusted pooled RD, random-effects meta-analysis).
The green dashed line indicates the prespecified non-inferiority margin of −1.3%.
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval; mRS: modified Rankin scale.
Figure 51.
Excellent functional outcome (mRS 0–1 at 90 days) in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 52.
Sensitivity analysis for excellent functional outcome (mRS 0–1 at 90 days) in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg, after additional inclusion of all patients returning to baseline mRS (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; mRS: modified Rankin scale.
Figure 53.
Pooled unadjusted common odds ratio for reduced disability (improvement of a least 1 point on the mRS at 90 days) in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled cOR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; cOdds Ratio: common odds ratio; CI: confidence interval; TE: treatment effect; SE: standard error.
Figure 54.
Major neurological improvement within 24 h in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Figure 55.
Pooled risk difference (in percent) for major neurological improvement within 24 h in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled RD, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; RD: risk difference; CI: confidence interval.
The rates of sICH according to the SITS-MOST definition, which was used by all studies included in this analysis, did not differ across treatment groups (OR = 0.50; 95%CI: 0.08–2.99; p = 0.45; I2 = 0%; Figure 56). The rates of any parenchymal haematoma with tenecteplase compared to alteplase were also similar (OR = 0.56; 95%CI: 0.09–3.75; p = 0.55; I2 = 57%; Figure 57). All-cause mortality at 3 months was similar between the two treatment groups (OR = 0.75; 95%CI: 0.49–1.13; p = 0.17; I2 = 0%; Figure 58).
Figure 56.
Symptomatic intracranial haemorrhage in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval; sICH: Symptomatic intracranial haemorrhage.
Figure 57.
Any parenchymal haematoma in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Figure 58.
All-cause mortality at 3 months in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
No difference in the onset-to-needle time was uncovered between the two treatment arms (mean difference = 1.8 min; 95%CI: −25.7 to 29.2 min; p = 0.90; I2 = 74%; Figure 59).
Figure 59.
Symptom onset-to-needle time (in minutes) in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (mean difference, random-effects meta-analysis).
With regards to neuroimaging parameters, rates of recanalisation before MT at first angiographic acquisition or averted mechanical thrombectomy were similar across treatment groups (OR = 1.49; 95%CI: 0.58–3.85; p = 0.41; I2 = 73%; Figure 60). The results of this meta-analysis do not corroborate the findings of EXTEND-IA TNK. 17 However, it should be noted that the primary endpoint of EXTEND-IA TNK was the rate of successful reperfusion before MT. 17 This trial only included patients with anterio circulation occlusions, and 75% of centres were MT-capable. In contrast, AcT was a pragmatic trial evaluating the safety and efficacy of IVT with tenecteplase in all AIS patients. Moreover, 94% of Act centres were comprehensive stroke centres with capability for MT. 21 Patients with both anterior and posterior circulation occlusions underwent MT. These disparities in patient and centre selection between EXTEND-IA TNK and AcT may account for the discrepant findings between these two trials. Rates of recanalisation within 24 h (irrespective of mechanical thrombectomy) did not differ between the two treatment groups (OR = 2.07; 95%CI: 0.87–4.96; p = 0.10; I2 = 49%; Figure 61).
Figure 60.
Recanalisation before mechanical thrombectomy at first angiographic acquisition or averted mechanical thrombectomy in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Figure 61.
Recanalisation within 24 h in patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration treated with intravenous thrombolysis with tenecteplase 0.25 mg/kg versus intravenous thrombolysis with alteplase 0.90 mg/kg (unadjusted pooled OR, random-effects meta-analysis).
TNK: tenecteplase; IV: inverse variance; CI: confidence interval.
Table 4 provides details regarding the assessment of the quality of evidence for all outcomes evaluated in PICO 2.
Table 4.
GRADE evidence profile for PICO 2.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | TNK (0.25 mg/kg) | alteplase (0.9mg/kg) | Relative (95% CI) | Absolute (95% CI) | ||
| Good functional outcome (modified Rankin Scale scores 0–2) at 90 days | ||||||||||||
| 3 | Randomised trials | Not serious | Serious a | Not serious | Not serious | None | 148/322 (46.0%) | 111/319 (34.8%) | OR 1.91 (1.05 to 3.48) | 157 more per 1000 (from 11 more to 302 more) | ⊕⊕⊕○Moderate | CRITICAL |
| Excellent functional outcome (modified Rankin Scale scores 0–1) at 90 days | ||||||||||||
| 4 | Randomised trials | Very seriousb,c | Not serious | Not serious | Not serious | None | 99/334 (30.5%) | 68/326 (21.3%) | OR 1.69 (1.15 to 2.47) | 100 more per 1000 (from 24 more to 186 more) | ⊕⊕○○Low | CRITICAL |
| Reduced disability (1 point or more reduction across all modified Rankin Scale scores) at 90 days | ||||||||||||
| 4 | Randomised trials | Very seriousb,c | Serious a | Not serious | Not serious | None | 357 (N/A) | 347 (N/A) | cOR 1.63 (1.05 to 2.54) | N/A | ⊕○○○Very low | CRITICAL |
| Mortality at 90 days | ||||||||||||
| 3 | Randomised trials | Serious c | Not serious | Not serious | Not serious | None | 49/322 (15.2%) | 62/319 (19.4%) | OR 0.75 (0.49 to 1.13) | 41 fewer per 1000 (from 89 fewer to 20 more) | ⊕⊕⊕○Moderate | CRITICAL |
| Symptomatic intracranial haemorrhage (sICH) at 24–48 h | ||||||||||||
| 2 | Randomised trials | Serious d | Not serious | Not serious | Serious e | None | 2/126 (1.6%) | 4/126 (3.2%) | OR 0.50 (0.08 to 2.99) | 16 fewer per 1000 (from 29 fewer to 58 more) | ⊕⊕○○Low | CRITICAL |
| Recanalisation before endovascular thrombectomy (EVT) at first angiographic acquisition or averted EVT | ||||||||||||
| 2 | Randomised trials | None | Serious a | Not serious | Serious e | None | 48/357 (13.4%) | 37/357 (10.4%) | OR 1.49 (0.58 to 3.85) | 43 more per 1000 (from 41 fewer to 204 more) | ⊕⊕○○Low | CRITICAL |
| Recanalisation within 24 h | ||||||||||||
| 3 | Randomised trials | Very seriousb,c,d | Not serious | Serious f | Serious e | None | 109/134 (81.3%) | 93/131 (71.0%) | OR 2.07 (0.87 to 4.96) | 125 more per 1000 (from 29 fewer to 214 more) | ⊕○○○Very low | CRITICAL |
| Onset-to-treatment time (min) | ||||||||||||
| 2 | Randomised trials | Serious d | Serious a | Not serious | Serious e | None | 126 | 126 | N/A | 1.8 min more (25.7 less to 29.2 more)* | ⊕○○○Very low | IMPORTANT |
| Major neurological improvement (according to definitions used in individual trials) at 24–72 h | ||||||||||||
| 2 | Randomised trials | Serious d | Serious a | Serious f | Serious e | None | 93/126 (73.8%) | 78/126 (61.9%) | OR 3.00 (0.39 to 23.11) | 211 more per 1000 (from 231 fewer to 355 more) | ⊕⊕○○Low | IMPORTANT |
| Any intracranial haemorrhage (ICH) | ||||||||||||
| 2 | Randomised trials | Serious d | Serious a | Not serious | Serious e | None | 7/126 (5.6%) | 10/126 (7.9%) | OR 0.56 (0.09 to 3.75) | 33 fewer per 1000 (from 73 fewer to 168 more) |
⊕○○○Very low | IMPORTANT |
TNK: tenecteplase, CI: confidence interval; MD: mean difference; OR: odds ratio; cOR: common odds ratio; N/A: not applicable.
Based on differences in pooled means (since there was no available data on medians with corresponding interquartile ranges in ⩾2 studies).
Presence of heterogeneity.
Inclusion of a post-hoc individual patient data meta-analysis of two RCTs.
Concerns due to lack of blinding in outcomes assessment in one study (ATTEST).
Evidence derived from less than half of the total studies population.
Wide and/or inconclusive confidence intervals.
Use of different outcome definitions across studies.

Additional information
A recent meta-analysis evaluated the safety and efficacy outcomes in LVO patients receiving IVT with either tenecteplase at different doses (0.10, 0.25 and 0.40 mg/kg) or alteplase at a standard dose of 0.90 mg/kg using RCT data. 50 Patients with LVO receiving tenecteplase had higher odds of good functional outcome (mRS 0–2; OR: 2.06, 95%CI = 1.15–3.69), successful recanalisation (OR = 3.05, 95%CI=1.73–5.40), and reduced disability (mRS shift analysis; cOR: 1.84, 95%CI: 1.18–2.87) at 3 months compared with patients with LVO receiving alteplase. In addition, observational evidence has also provided preliminary indirect and direct evidence suggesting that pretreatment with tenecteplase in patients with LVO eligible for MT may be associated with faster recanalisation, higher rates of successful recanalisation and improved early clinical outcomes compared to alteplase treatment.42,44–46

PICO 3 Wake-up stroke/unknown onset
3.1 For patients with acute ischaemic stroke on awakening from sleep or acute ischaemic stroke of unknown onset and who are eligible for intravenous thrombolysis, does intravenous thrombolysis with tenecteplase 0.25 mg/kg compared with no intravenous thrombolysis lead to:
(a) a non-inferior proportion of patients with excellent functional outcome (mRS scores of 0–1) at 90 days?
(b) non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, good functional outcome defined by mRS 0–2 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)?
(c) a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)?
(d) a reduction in key time metrics (onset-to-treatment time, door-to-needle time)?
(e) an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth within the first 24 h)?
Analysis of current evidence
The literature search identified one completed RCT addressing this PICO question.
TWIST 50 (Tenecteplase in Wake-up Ischaemic Stroke Trial) was a pragmatic phase 3 RCT with PROBE design whose results were recently presented at ESOC 2022. TWIST randomised 578 AIS patients with wake-up stroke selected by non-contrast CT only, who had no other contraindications to IVT administration and could receive IVT treatment within 4.5 h from awakening, into two treatment arms: tenecteplase 0.25 mg/kg plus standard care versus standard care alone without thrombolysis with tenecteplase or any other thrombolytic agent. The primary endpoint was the distribution of mRS scores on the ordinal scale at 3 months (shift analysis). The adjusted cOR for reduced disability was 1.18 (95%CI: 0.88–2.58); p = 0.27). Additionally, excellent functional outcome (mRS 0–1) at 3 months did not differ between tenecteplase-treated patients (45.1%) versus controls (38.3%; adjusted OR: 1.33; 95%CI: 0.94–1.87; p = 0.10). No safety issues emerged with tenecteplase treatment compared to no IVT, including sICH (adjusted OR: 3.12; 95%CI = 0.83–11.70), any ICH (adjusted OR: 1.14; 95%CI = 0.67–1.94), parenchymal haematoma type 2 (adjusted OR: 1.47; 95%CI = 0.46–4.73) and 3-month mortality (adjusted OR: 1.29; 95%CI = 0.74–2.26). In conclusion, TWIST did not provide evidence that IVT with tenecteplase compared to standard of care improved functional outcomes in AIS patients with wake-up stroke selected with non-contrast CT. No other randomised or observational data were available with regard to this PICO question.
Table 5 provides details regarding the assessment of the quality of evidence for all outcomes evaluated in PICO 3.1.
Table 5.
GRADE evidence profile for PICO 3.1.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | TNK (0.25 mg/ kg) | Alteplase (0.9 mg/ kg) | Relative (95% CI) | Absolute (95% CI) | ||
| Excellent functional outcome (modified Rankin Scale scores 0–1) at 90 days | ||||||||||||
| 1 | Randomised trial | Unclear a | Not serious | Not serious | Serious b | Evidence provided by a single phase 2 trial | 130/288 (45.1%) | 111/290 (38.3%) | OR 1.33 (0.95 to 1.85) | 69 more per 1000 (from 12 fewer to 152 more) | ⊕⊕○○Low | CRITICAL |
| Reduced disability (1 point or more reduction across all modified Rankin Scale scores) at 90 days | ||||||||||||
| 1 | Randomised trial | Unclear a | Not serious | Not serious | Serious b | Evidence provided by a single phase 2 trial | N/A | N/A | cOR 1.18 (0.88 to 1.58) | 1 fewer per 1000 (from 2 fewer to 1 fewer) | ⊕⊕○○Low | CRITICAL |
| Symptomatic intracranial haemorrhage (sICH) within 36 h | ||||||||||||
| 1 | Randomised trial | Unclear a | Not serious | Not serious | Serious b | Evidence provided by a single phase 2 trial | 9/288 (3.1%) | 3/290 (1.0%) | OR 3.09 (0.83 to 11.52) | 21 more per 1000 (from 2 fewer to 97 more) | ⊕⊕○○Low | CRITICAL |
| Any intracranial haemorrhage (ICH) | ||||||||||||
| 1 | Randomised trial | Unclear a | Not serious | Not serious | Serious b | Evidence provided by a single phase 2 trial | 33/288 (11.5%) | 30/290 (10.3%) | OR 1.12 (0.66 to 1.89) | 11 more per 1000 (from 33 fewer to 76 more) | ⊕⊕○○Low | IMPORTANT |
| Mortality at 90 days | ||||||||||||
| 1 | Randomised trial | Unclear a | Not serious | Not serious | Serious b | Evidence provided by a single phase 2 trial | 28/288 (9.7%) | 23/290 (7.9%) | OR 1.25 (0.70 to 2.23) | 18 more per 1000 (from 22 fewer to 82 more) | ⊕⊕○○Low | CRITICAL |
| Onset to treatment time (min) | ||||||||||||
| 1 | Randomised trial | Unclear a | Not serious | Not serious | Serious b | Evidence provided by a single phase 2 trial | 288 | 290 | N/A | MD 1.6 min less (12.8 less to 9.6 more) | ⊕⊕○○Low | IMPORTANT |
TNK: tenecteplase, CI: confidence interval; MD: mean difference; OR: odds ratio; cOR: common odds ratio; N/A: not applicable.
Study not published to date.
Wide and/or inconclusive confidence intervals.

Additional information
According to the ESO guidelines, intravenous thrombolysis is recommended for patients with AIS on awakening from sleep or AIS of unknown onset that are selected by certain advanced neuroimaging criteria, that is, either presenting DWI/FLAIR mismatch or having CT or MRI core/perfusion mismatch. 2 Selection of patients and IVT administration in the extended time window (>4.5 h from symptom onset) based on non-contrast CT only is not recommended.
3.2 For patients with acute ischaemic stroke on awakening from sleep or acute ischaemic stroke of unknown onset and who are eligible for intravenous thrombolysis, does intravenous thrombolysis with tenecteplase 0. or 0.40 mg/kg compared with intravenous thrombolysis with alteplase 0.90 mg/kg lead to:
(a) a non-inferior proportion of patients with excellent functional outcome (mRS scores of 0–1) at 90 days?
(b) non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, good functional outcome defined by mRS 0–2 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)?
(c) a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)?
(d) a reduction in key time metrics (onset-to-treatment time, door-to-needle time)?
(e) an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth within the first 24 h)?
Analysis of current evidence
There are no dedicated RCTs addressing this PICO question.
A post-hoc analysis 51 of NOR-TEST 14 sought to investigate the safety and efficacy of IVT with tenecteplase 0.40 mg/kg compared to alteplase (0.90 mg/kg) in wake-up stroke patients. Included patients were treated based on FLAIR-DWI mismatch. More specifically, among 40 wake-up stroke patients analysed, there was no difference with regard to excellent functional outcome at 3 months between patients randomised to alteplase (65.2%) and tenecteplase (68.8%). No case of sICH or death were observed in either tenecteplase or alteplase treated patients. There was a higher rate of major neurological improvement within the first 24 h in the tenecteplase- versus alteplase-treated arms (87.5% vs 54.2%, p = 0.027). However, the patient population in this study was very limited and included patients with mostly minor stroke syndromes (median baseline NIHSS-score 4.5 points). 51
No other randomised or observational data were available with regard to this PICO.


Discussion
This expedited recommendation was developed following the GRADE methodology and aims to assist physicians in decision-making regarding the use of intravenous tenecteplase in patients with acute ischaemic stroke eligible for IVT. 23 It includes up-to-date evidence that supersedes the tenecteplase sections of the 2021 ESO Guidelines on IVT 2 and the 2019 ESO-ESMINT Guidelines on MT. 27 A summary of PICO questions, evidence-based recommendations, and expert consensus statements is provided in Table 6.
Table 6.
Synoptic table of all recommendations and expert consensus statements.
| Topic/PICO Question | Recommendation | Expert consensus statement |
|---|---|---|
|
PICO 1 (AIS patients <4.5 h).
1.1 For patients with acute ischaemic stroke of <4.5 h duration, does intravenous thrombolysis with tenecteplase 0.25 mg/kg compared with intravenous thrombolysis with alteplase 0.90 mg/kg lead to: |
For patients with acute ischaemic stroke of <4.5 h duration who are eligible for intravenous thrombolysis, tenecteplase 0.25 mg/kg can be used as a safe and effective alternative to alteplase 0.9 mg/kg. | All MWG members suggest favouring tenecteplase 0.25 mg/kg over alteplase 0.9 mg/kg for patients with acute ischaemic stroke of <4.5 h duration in light of safety and efficacy data and because tenecteplase can be administered with a single bolus rather than a 1-hr infusion. Voting: 9/9 members. |
| - a non-inferior proportion of patients with excellent functional outcome (mRS scores of 0–1) at 90 days? |
Quality of evidence: Moderate ⊕⊕⊕
Strength of recommendation: Strong ↑↑ |
|
| - non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, good functional outcome defined by mRS 0–2 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)? | ||
| - a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)? | ||
| - a reduction in key time metrics (onset-to-treatment time, door-to-needle time)? | ||
| - an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth within the first 24 h)? | ||
| 1.2 For patients with acute ischaemic stroke of <4.5 h duration, does intravenous thrombolysis with tenecteplase 0.40 mg/kg compared with intravenous thrombolysis with alteplase 0.90 mg/kg lead to: | For patients with acute ischaemic stroke of <4.5 h duration who are eligible for intravenous thrombolysis, we recommend against using tenecteplase at a dose of 0.40 mg/kg. | |
| - a non-inferior proportion of patients with excellent functional outcome (mRS scores of 0–1) at 90 days? - non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, good functional outcome defined by mRS 0–2 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)? |
Quality of evidence: Low ⊕⊕
Strength of recommendation: Strong against intervention ↓↓ |
|
| - a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)? | ||
| - a reduction in key time metrics (onset-to-treatment time, door-to-needle time)? | ||
| - an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth within the first 24 h)? | ||
| 1.3 In patients with acute ischaemic stroke of <4.5 h duration with prehospital management with a mobile stroke unit does intravenous thrombolysis with tenecteplase 0.25 mg/kg compared with intravenous thrombolysis with alteplase 0.90 mg/kg lead to: | For patients with acute ischaemic stroke of <4.5 h duration with prehospital management with a mobile stroke unit who are eligible for intravenous thrombolysis, we suggest tenecteplase 0.25 mg/kg over alteplase 0.90 mg/kg. | |
| - a non-inferior proportion of patients with excellent functional outcome (mRS scores of 0–1) at 90 days? |
Quality of evidence: Low ⊕⊕
Strength of recommendation : Weak ↑? |
|
| - non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, good functional outcome defined by mRS 0–2 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)? | ||
| - a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)? | ||
| - a reduction in key time metrics (onset-to-treatment time, door-to-needle time)? - an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth within the first 24 h)? |
||
|
PICO 2 (Patients <4.5 h and large vessel occlusion).
2.1 For large vessel occlusion acute ischaemic stroke patients of <4.5 h duration does intravenous thrombolysis with tenecteplase 0.25 mg/kg compared with intravenous thrombolysis with alteplase 0.90 mg/kg lead to: |
For patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration who are eligible for intravenous thrombolysis, we recommend tenecteplase 0.25 mg/kg over alteplase 0.9 mg/kg. Intravenous thrombolysis should not delay mechanical thrombectomy. Quality of evidence: Moderate ⊕⊕⊕ Strenght of recommendation: Strong ↑↑ |
For patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration who are eligible for intravenous thrombolysis and are directly admitted to a thrombectomy-capable centre, all MWG members suggest IVT with tenecteplase 0.25 mg/kg over skipping IVT. For patients with large vessel occlusion acute ischaemic stroke of <4.5 h duration who are eligible for intravenous thrombolysis and are admitted to a centre without mechanical thrombectomy capability, all MWG members suggest IVT with tenecteplase 0.25 mg/kg followed by rapid transfer to a thrombectomy-capable centre. Voting: 9/9 members. |
| - a non-inferior proportion of patients with good functional outcome (mRS scores of 0–2) at 90 days? | ||
| - non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, excellent functional outcome defined by mRS 0–1 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)? | ||
| - a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)? | ||
| - a reduction in key time metrics (onset-to-treatment time, door-to-needle time)? | ||
| - an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth at 24 h, recanalisation at 24 h, recanalisation at the end of mechanical thrombectomy, recanalisation before mechanical thrombectomy at first angiographic acquisition or averted mechanical thrombectomy)? | ||
|
PICO 3 (Wake-up stroke/unknown onset).
3.1 For patients with acute ischaemic stroke on awakening from sleep or acute ischaemic stroke of unknown onset and who are eligible for intravenous thrombolysis, does intravenous thrombolysis with tenecteplase 0.25 mg/kg compared with no intravenous thrombolysis lead to: |
For patients with acute ischaemic stroke on awakening from sleep or acute ischaemic stroke of unknown onset who are selected with non-contrast CT, we recommend against intravenous thrombolysis with tenecteplase 0.25 mg/kg. | All MWG members suggest that tenecteplase 0.25 mg/kg could be a reasonable alternative to alteplase 0.9 mg/kg for patients with acute ischaemic stroke on awakening from sleep or acute ischaemic stroke of unknown onset and who are eligible for intravenous thrombolysis after selection with advanced imaging (FLAIR-DWI mismatch or Perfusion mismatch as outlined in the 2021 ESO Guidelines on IVT). Voting: 9/9 members |
|
Quality of evidence: Low ⊕⊕
Strength of recommendation: Strong against intervention ↓↓ For patients with acute ischaemic stroke on awakening from sleep or acute ischaemic stroke of unknown onset and who are eligible for intravenous thrombolysis, there is continued uncertainty over the potential benefits and harms of tenecteplase compared with alteplase. Quality of evidence: Very low ⊕ Strength of recommendation: - | ||
| - a non-inferior proportion of patients with excellent functional outcome (mRS scores of 0–1) at 90 days? | ||
| - non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, good functional outcome defined by mRS 0–2 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)? | ||
| - a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)? | ||
| - a reduction in key time metrics (onset-to-treatment time, door-to-needle time)? - an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume |
||
| at 24 h, ischaemic core growth within the first 24 h)? | ||
| 3.2 For patients with acute ischaemic stroke on | ||
| awakening from sleep or acute ischaemic stroke | ||
| of unknown onset and who are eligible for intravenous thrombolysis, does intravenous thrombolysis with tenecteplase 0.25 mg/kg or 0.40 mg/kg compared with intravenous thrombolysis with alteplase 0.90 mg/kg lead to: | ||
| - a non-inferior proportion of patients with excellent functional outcome (mRS scores of 0–1) at 90 days? | ||
| - non-inferior or better results on other efficacy outcomes (mRS shift analysis at 90 days, good functional outcome defined by mRS 0–2 at 90 days, major neurological improvement at 24–72 h, improved quality of life metrics)? | ||
| - a reduction in the risk of adverse events (mortality at 90 days, symptomatic intracranial haemorrhage, any intracranial haemorrhage, any parenchymal haematoma, extracranial bleeding)? | ||
| - a reduction in key time metrics (onset-to-treatment time, door-to-needle time)? | ||
| - an improvement in neuroimaging parameters (reperfusion at 24 h, final infarct volume at 24 h, ischaemic core growth within the first 24 h)? |
Potential theoretical benefits of IVT with tenecteplase compared to alteplase might include efficacy (better functional outcome in patients with LVO, potentially faster and higher reperfusion rates), safety (reduction of the risks of sICH, systemic bleeding and mortality rates), and logistical improvements (lack of 1-h infusion, time metrics reduction).52,53 For functional outcome, we used in our recommendation an absolute non-inferiority margin of 3% that would preserve at least half of the conservative estimate (lower 95%CI limit) of alteplase effect compared with placebo in AIS patients, 1 which was also used in recently published RCTs.1,33 However, since non-inferiority is always a trade-off between the estimated effect one is prepared to sacrifice and the expected benefits of the experimental treatment, we added a secondary analysis with a more stringent 1.3% margin, which was derived in a survey of U.S. stroke neurologists. 35
We decided to provide separate recommendations for patients with AIS treated within 4.5 h (PICO 1), AIS patients with LVO (PICO 2), and patients with AIS of unknown onset or awakening from sleep (PICO 3). This decision was based on several reasons. First, similar distinctions were made in previous recommendations, 2 as those patients differ in severity, functional outcome, and correspond to different clinical scenarios. Secondly, there are accruing data indicating potentially higher rates of successful reperfusion with tenecteplase compared to alteplase in this specific AIS subgroup with LVO. 25 Finally, unknown onset/wake-up strokes require a distinct diagnostic approach including advanced imaging.
In a general population of AIS patients treated within 4.5 h, we found moderate quality evidence that tenecteplase is non-inferior to alteplase in terms of excellent functional outcome, and low quality evidence that there were no significant differences in terms of sICH, mortality, and treatment time metrics. However, it should be noted that the rates of any intracranial haemorrhage were lower with tenecteplase than alteplase. Contrary to previous recommendations, 2 we can now recognise 0.25 mg/kg tenecteplase as a promising alternative to alteplase in all AIS treated within 4.5 h. This new recommendation is supported notably by the recent AcT Trial, the largest RCT with a total of 1600 AIS patients comparing tenecteplase with alteplase. One of the expected benefits of tenecteplase is its ease of use that could result in shorter treatment times and less staffing resources in the emergency setting. However, in RCTs other factors such as the inclusion and randomisation times can interfere and may diminish a possible effect.
In AIS patients with LVO treated within 4.5 h, we found moderate quality evidence that tenecteplase is superior to alteplase in terms of good functional outcome, and low quality evidence that there were no significant differences in terms of sICH. This strengthens our previous recommendation that 0.25 mg/kg tenecteplase may be preferable compared to alteplase in AIS patients with LVO treated within 4.5 h. 2 One hypothesis as to how this functional improvement could be mediated is a higher and/or earlier recanalisation with tenecteplase than alteplase because of pharmacologic properties.52,54,55 However, in the two RCTs that reported recanalisation rates before MT, we found heterogeneous results with a 10% rate for both tenecteplase and alteplase in AcT, and a 22% rate with tenecteplase versus 10% with alteplase in EXTEND- IA TNK. 11 EXTEND-IA TNK was designed to address the specific question of recanalisation, contrary to AcT. In addition, EXTEND-IA TNK randomised less patients in ‘mothership’ settings than AcT, respectively 75% and 94%. Hence, it remains unclear whether tenecteplase actually improves pre-MT recanalisation, and how it can lead to better functional outcome. Notably, it should be noted that although patients with basilar artery occlusions were included in both EXTEND-IA TNK and AcT, they represented a minority (⩽5%) of enrolled patients.
Finally, there was low quality evidence that tenecteplase is not superior to no IVT for AIS of unknown onset. 50 However, this result is based on the results of only one RCT that did not use advanced neuroimaging in patient selection. Additionally, there have been no published RCTs on the use of tenecteplase in extended time windows. However, several ongoing studies should provide more data on these patients in the coming years: Randomisation to Extend Stroke Intravenous ThromboLysis In Evolving Non-Large Vessel Occlusion With TNK (RESILIENT EXTEND-IV, NCT05199662); Extending the Time Window for Tenecteplase by Effective Reperfusion in Patients With Large Vessel Occlusion (ETERNAL-LVO, NCT04454788); Extending the Time Window for Tenecteplase by Recanalisation of Basilar Artery Occlusion in Posterior Circulation Stroke (POST-ETERNAL, NCT05105633); A Randomised Controlled Trial of TNK-tPA Versus Standard of Care for Minor Ischaemic Stroke With Proven Occlusion (TEMPO-2, NCT02398656); Tenecteplase in Stroke Patients Between 4.5 and 24 Hours (TIMELESS, NCT03785678). Additionally, there are several ongoing RCTs comparing Tenecteplase 0.25 mg/kg and alteplase for AIS patients within 4.5 h: Tenecteplase versus Alteplase for Stroke Thrombolysis Evaluation-2 (ATTEST-2, NCT02814409); Tenecteplase versus Alteplase for Stroke Thrombolysis Evaluation for patients with CT perfusion penumbra (TASTE-B, ACTRN12613000243718); Norwegian Tenecteplase Stroke Trial 2 (NORTEST 2-Part B). All these under way RCTs will be very useful to increase the quality of evidence of our recommendations that are graded as low or very low for some of the recommendations. Enrolling patients in a dedicated RCT is strongly recommended to further clarify the safety and efficacy of tenecteplase for unselected AIS and LVO patients. In addition, future studies comparing tenecteplase 0.25 mg/kg and alteplase in patients with unknown symptom onset time selected with advanced imaging are also needed.
While waiting for the results of these trials, to support physicians in their practical decision-making, expert consensus statements are provided in a dedicated paragraph. A perfect agreement among experts in favour of tenecteplase was obtained for the patients with AIS of <4.5 h duration (9/9), with LVO AIS of <4.5 h duration before MT (9/9), and with AIS on awakening or of unknown onset (9/9).
After the TNK-S2B trial that assessed three doses of 0.10, 0.25 and 0.40 mg/kg (maximum 10, 20 and 40 mg respectively), two doses (0.25 and 0.40 mg/kg) have been tested in several RCTs in patients with AIS. In our analysis, we found low quality evidence that 0.40 mg/kg tenecteplase crossed the non-inferior thresholds compared to alteplase for functional outcome (excellent or good). Additionally, the EXTEND-IA TNK Part 2 trial which compared both 0.25 and 0.40 mg/kg doses of tenecteplase in patients with LVO did not find any benefit with the higher dose. 18 Taken with the trend towards a numerically higher sICH rates with 0.40 mg/kg tenecteplase both in NOR-TEST 2 Part A and EXTEND-IA TNK Part 2 (although non statistically significant), we suggest that there is sufficient data to support the exclusive use of the 0.25 mg/kg dose of tenecteplase in AIS. It is important to note that the only currently available packaging of tenecteplase, was designed for the treatment of acute myocardial infarction, and therefore has a weight-based graduated syringe corresponding to a dosage of 0.5 mg/kg. Precautions about this packaging are necessary to avoid dosing errors in AIS patients.
A main safety criterion in AIS management is the occurrence of sICH. However, analysis across studies for this item is limited by the lack of one common sICH definition. For instance, among the seven studies focusing on unselected AIS patients treated within 4.5 h, only four used a common (SITS-MOST) definition. In order to address the inconsistencies in sICH definitions, we performed an analysis which included all studies, using each study’s definition, and a second analysis which was restricted to the RCTs that used the same definition.
Although the use of intravenous tenecteplase remains off-label, our recommendations open the way for a broader use of tenecteplase in AIS patients. However, we acknowledge that their implementation will be drastically limited by the tenecteplase shortages experienced currently in Europe. The use of alteplase currently represents the standard of care with also substantial shortages in the supply chain in Europe, however its comparative efficacy with tenecteplase may be questioned in AIS patients with LVO. We hope that the pharmaceutical industry and European regulators may provide a swift and effective solution to improve thrombolytics supply and hopefully expand the tenecteplase label for AIS, with appropriate packaging for the 0.25 mg/kg dose.
Plain language summary
Individuals who suffer a stroke from a clot blocking the vessels in the brain can be treated by injecting clot-dissolving drugs into a vein in the arm, a procedure called intravenous thrombolysis. Intravenous thrombolysis started within 4.5 h from the onset of stroke symptoms improves breaking down of the clots in the brain and allows improvement of symptoms. The most used clot-busting medication used in patients with stroke is called alteplase. However, there is a newer thrombolytic drug called tenecteplase. Tenecteplase is used to treat heart attacks, and has gained interest among stroke doctors in recent years. The dose of tenecteplase that is used to treat people with stroke is calculated based on their weight in kg. This document provides recommendations on using tenecteplase instead of alteplase to treat patients with stroke.
Tenecteplase at a dose of 0.25 mg/kg is equally safe and effective to alteplase for the treatment of patients presenting with stroke symptoms within 4.5 h.
Tenecteplase should not be used at a higher dose for stroke treatment.
Tenecteplase at a dose of 0.25 mg/kg may be better than alteplase for patients treated in a specialised ambulance capable of performing brain imaging.
Patients with stroke due to a blood clot in a large artery in the brain should be treated with tenecteplase at a dose of 0.25 mg/kg rather than alteplase, prior to receiving an intervention to remove the clot out of the body (thrombectomy).
For patients becoming aware of stroke symptoms on awakening from sleep or those presenting without information on the time of symptoms onset clot-busting medications can only be given if access to special brain imaging is available. In this setting we do not know whether tenecteplase can be used instead of alteplase. However, the opinion of the experts writing the current document is that tenecteplase at a dose of 0.25 mg/kg may be used instead of alteplase in patients becoming aware of stroke symptoms on awakening from sleep or those presenting without information on the time of symptoms onset, provided they meet certain criteria on special brain imaging.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873221150022 for European Stroke Organisation (ESO) expedited recommendation on tenecteplase for acute ischaemic stroke by Sonia Alamowitch, Guillaume Turc, Lina Palaiodimou, Andrew Bivard, Alan Cameron, Gian Marco De Marchis, Annette Fromm, Janika Kõrv, Melinda B Roaldsen, Aristeidis H Katsanos and Georgios Tsivgoulis in European Stroke Journal
Acknowledgments
The authors would like to thank Dr. Gaspard Gerschenfeld for useful discussion.Dr. Katsanos is supported by an internal career award from the Department of Medicine, McMaster University.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Intellectual and financial disclosures of the module working group members are presented in Supplemental Table.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Not applicable.
Informed consent: Not applicable.
Guarantor: SA
Contributorship: Sonia Alamowitch, Georgios Tsivgoulis and Guillaume Turc wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript. Lina Palaiodimou, Aristeidis Katsanos and Georgios Tsivgoulis conducted the statistical analyses.
ORCID iDs: Sonia Alamowitch  https://orcid.org/0000-0002-0074-3520
https://orcid.org/0000-0002-0074-3520
Guillaume Turc  https://orcid.org/0000-0001-5059-4095
https://orcid.org/0000-0001-5059-4095
Lina Palaiodimou  https://orcid.org/0000-0001-7757-609X
https://orcid.org/0000-0001-7757-609X
Alan Cameron  https://orcid.org/0000-0001-6965-1109
https://orcid.org/0000-0001-6965-1109
Gian Marco De Marchis  https://orcid.org/0000-0002-0342-9780
https://orcid.org/0000-0002-0342-9780
Janika Kõrv  https://orcid.org/0000-0002-6074-0727
https://orcid.org/0000-0002-6074-0727
Georgios Tsivgoulis  https://orcid.org/0000-0002-0640-3797
https://orcid.org/0000-0002-0640-3797
Supplemental material: Supplemental material for this article is available online.
References
- 1. Emberson J, Lees KR, Lyden P, et al. . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berge E, Whiteley W, Audebert H, et al. . European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021; 6: I–LXII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thiebaut AM, Gauberti M, Ali C, et al. . The role of plasminogen activators in stroke treatment: fibrinolysis and beyond. Lancet Neurol 2018; 17: 1121–1132. [DOI] [PubMed] [Google Scholar]
- 4. Tsivgoulis G, Kargiotis O, De Marchis G, et al. . Off-label use of intravenous thrombolysis for acute ischemic stroke: a critical appraisal of randomized and real-world evidence. Ther Adv Neurol Disord 2021; 14: 1756286421997368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators, Van de Werf F. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet 1999; 354: 716–722. [DOI] [PubMed] [Google Scholar]
- 6. Neumann F-J, Sousa-Uva M, Ahlsson A, et al. . 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87–165. [DOI] [PubMed] [Google Scholar]
- 7. Thomas GR, Thibodeaux H, Errett CJ, et al. . A long-half-life and fibrin-specific form of tissue plasminogen activator in rabbit models of embolic stroke and peripheral bleeding. Stroke 1994; 25: 2072–2079. [DOI] [PubMed] [Google Scholar]
- 8. Chapman DF, Lyden P, Lapchak PA, et al. . Comparison of TNK with wild-type tissue plasminogen activator in a rabbit embolic stroke model. Stroke 2001; 32: 748–752. [DOI] [PubMed] [Google Scholar]
- 9. Haley EC, Lyden PD, Johnston KC, et al. . A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke 2005; 36: 607–612. [DOI] [PubMed] [Google Scholar]
- 10. Coutts SB, Dubuc V, Mandzia J, et al. . Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke 2015; 46: 769–774. [DOI] [PubMed] [Google Scholar]
- 11. Warach SJ, Dula AN, Milling TJ, et al. . Prospective observational cohort study of tenecteplase versus alteplase in routine clinical practice. Stroke 2022; 53: 3583–3593. [DOI] [PubMed] [Google Scholar]
- 12. Gerschenfeld G, Liegey JS, Laborne FX, et al. . Treatment times, functional outcome, and hemorrhage rates after switching to tenecteplase for stroke thrombolysis: insights from the TETRIS registry. Eur Stroke J 2022; 7: 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haley EC, Thompson JL, Grotta JC, et al. .; Tenecteplase in Stroke Investigators. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke 2010; 41: 707–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Logallo N, Novotny V, Assmus J, et al. . Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol 2017; 16: 781–788. [DOI] [PubMed] [Google Scholar]
- 15. Huang X, Cheripelli BK, Lloyd SM, et al. . Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol 2015; 14: 368–376. [DOI] [PubMed] [Google Scholar]
- 16. Parsons M, Spratt N, Bivard A, et al. . A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med 2012; 366: 1099–1107. [DOI] [PubMed] [Google Scholar]
- 17. Campbell BCV, Mitchell PJ, Churilov L, et al. .; EXTEND-IA TNK Investigators. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med 2018; 378: 1573–1582. [DOI] [PubMed] [Google Scholar]
- 18. Campbell BCV, Mitchell PJ, Churilov L, et al. . Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke. JAMA 2020; 323: 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li S, Pan Y, Wang Z, et al. . Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol 2022; 7: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bivard A, Zhao H, Churilov L, et al. . Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile Stroke Unit (TASTE-A): a phase 2, randomised, open-label trial. Lancet Neurol 2022; 21: 520–527. [DOI] [PubMed] [Google Scholar]
- 21. Sajobi T, Singh N, Almekhlafi MA, et al. .; AcT Trial Investigators. Alteplase compared to tenecteplase in patients with acute ischemic stroke (AcT) trial: Protocol for a Pragmatic Registry linked randomized clinical trial. Lancet 2022; 400: 161–169. [DOI] [PubMed] [Google Scholar]
- 22. Kvistad CE, Næss H, Helleberg BH, et al. . Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol 2022; 21: 511–519. [DOI] [PubMed] [Google Scholar]
- 23. Steiner T, Dichgans M, Norrving B, et al. . European Stroke Organisation (ESO) standard operating procedure for the preparation and publishing of guidelines. Eur Stroke J 2021; 6: CXXII–CXXXIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyatt GH, Oxman AD, Schünemann HJ, et al. . GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011; 64: 380–382. [DOI] [PubMed] [Google Scholar]
- 25. Katsanos AH, Safouris A, Sarraj A, et al. . Intravenous thrombolysis with tenecteplase in patients with large vessel occlusions: systematic review and meta-analysis. Stroke 2021; 52: 308–312. [DOI] [PubMed] [Google Scholar]
- 26. Saver JL, Chaisinanunkul N, Campbell BCV, et al. . Standardized Nomenclature for Modified Rankin Scale Global Disability Outcomes: Consensus Recommendations from Stroke Therapy Academic Industry Roundtable XI. Stroke 2021; 52: 3054–3062. [DOI] [PubMed] [Google Scholar]
- 27. Turc G, Tsivgoulis G, Audebert HJ, et al. . European Stroke Organisation - European Society for minimally Invasive Neurological Therapy expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischaemic stroke and anterior circulation large vessel occlusion. Eur Stroke J 2022; 7: I–XXVI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walter S, Audebert HJ, Katsanos AH, et al. . European Stroke Organisation (ESO) guidelines on mobile stroke units for prehospital stroke management. Eur Stroke J 2022; 7: XXVII–LIX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higashida RT, Furlan AJ, Roberts H, et al. . Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 30. Katsanos AH, Malhotra K, Goyal N, et al. . Intravenous thrombolysis prior to mechanical thrombectomy in large vessel occlusions. Ann Neurol 2019; 86: 395–406. [DOI] [PubMed] [Google Scholar]
- 31. Sterne JAC, Savović J, Page MJ, et al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 32. Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- 33. Saver JL, Mistry E. Time to recognize three classes of non-inferiority trial margins. J Neurointerv Surg 2023; 15: 2–4. [DOI] [PubMed] [Google Scholar]
- 34. Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials 2011; 12: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cranston JS, Kaplan BD, Saver JL. Minimal clinically important difference for safe and simple novel acute ischemic stroke therapies. Stroke 2017; 48: 2946–2951. [DOI] [PubMed] [Google Scholar]
- 36. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 37. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke 2019; 50: 2156–2162. [DOI] [PubMed] [Google Scholar]
- 38. Nepal G, Kharel G, Ahamad ST, et al. . Tenecteplase versus alteplase for the management of acute ischemic stroke in a low-income Country–Nepal: cost, efficacy, and safety. Cureus 2018; 10: e2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beharry J, Waters MJ, Drew R, et al. . Dabigatran reversal before intravenous tenecteplase in acute ischemic stroke. Stroke 2020; 51: 1616–1619. [DOI] [PubMed] [Google Scholar]
- 40. Mahawish K, Gommans J, Kleinig T, et al. . Switching to tenecteplase for stroke thrombolysis: real-world experience and outcomes in a regional stroke network. Stroke 2021; 52: e590–e593. [DOI] [PubMed] [Google Scholar]
- 41. Zhong CS, Beharry J, Salazar D, et al. . Routine use of tenecteplase for thrombolysis in acute ischemic stroke. Stroke 2021; 52: 1087–1090. [DOI] [PubMed] [Google Scholar]
- 42. Psychogios K, Palaiodimou L, Katsanos AH, et al. . Real-world comparative safety and efficacy of tenecteplase versus alteplase in acute ischemic stroke patients with large vessel occlusion. Ther Adv Neurol Disord 2021; 14: 1756286420986727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gerschenfeld G, Smadja D, Turc G, et al. .; TETRIS Study Group. Functional outcome, recanalization, and hemorrhage rates after large vessel occlusion stroke treated with tenecteplase before thrombectomy. Neurology 2021; 97: e2173–e2184. [DOI] [PubMed] [Google Scholar]
- 44. Alemseged F, Ng FC, Williams C, et al. .; BATMAN study group and EXTEND IA TNK study group. Tenecteplase vs alteplase before endovascular therapy in basilar artery occlusion. Neurology 2021; 96: e1272–e1277. [DOI] [PubMed] [Google Scholar]
- 45. Tsivgoulis G, Katsanos AH, Christogiannis C, et al. . Intravenous thrombolysis with tenecteplase for the treatment of acute ischemic stroke. Ann Neurol 2022; 92: 349–357. [DOI] [PubMed] [Google Scholar]
- 46. Katsanos AH, Psychogios K, Turc G, et al. . Off-Label use of tenecteplase for the treatment of acute ischemic stroke: A systematic review and meta-analysis. JAMA Netw Open 2022; 5: e224506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kheiri B, Osman M, Abdalla A, et al. . Tenecteplase versus alteplase for management of acute ischemic stroke: a pairwise and network meta-analysis of randomized clinical trials. J Thromb Thrombolysis 2018; 46: 440–450. [DOI] [PubMed] [Google Scholar]
- 48. Jacob AP, Parker SA, Bowry R, et al. . How frequent is the one-hour tPA infusion interrupted or delayed? J Stroke Cerebrovasc Dis 2022; 31: 106471. [DOI] [PubMed] [Google Scholar]
- 49. Bivard A, Huang X, Levi CR, et al. . Tenecteplase in ischemic stroke offers improved recanalization: analysis of 2 trials. Neurology 2017; 89: 62–67. [DOI] [PubMed] [Google Scholar]
- 50. Roaldsen MB, Lindekleiv H, Eltoft A, et al. . Tenecteplase in wake-up ischemic stroke trial: protocol for a randomized-controlled trial. Int J Stroke 2021; 16: 990–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ahmed HK, Logallo N, Thomassen L, et al. . Clinical outcomes and safety profile of tenecteplase in wake-up stroke. Acta Neurol Scand 2020; 142: 475–479. [DOI] [PubMed] [Google Scholar]
- 52. Marshall RS. Progress in intravenous thrombolytic therapy for acute stroke. JAMA Neurol 2015; 72: 928–934. [DOI] [PubMed] [Google Scholar]
- 53. Tanswell P, Modi N, Combs D, et al. . Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet 2002; 41: 1229–1245. [DOI] [PubMed] [Google Scholar]
- 54. Putaala J, Saver JL, Nour M, et al. . Should tenecteplase be given in clinical practice for acute ischemic stroke thrombolysis? Stroke 2021; 52: 3075–3080. [DOI] [PubMed] [Google Scholar]
- 55. Safouris A, Magoufis G, Tsivgoulis G. Emerging agents for the treatment and prevention of stroke: progress in clinical trials. Expert Opin Investig Drugs 2021; 30: 1025–1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873221150022 for European Stroke Organisation (ESO) expedited recommendation on tenecteplase for acute ischaemic stroke by Sonia Alamowitch, Guillaume Turc, Lina Palaiodimou, Andrew Bivard, Alan Cameron, Gian Marco De Marchis, Annette Fromm, Janika Kõrv, Melinda B Roaldsen, Aristeidis H Katsanos and Georgios Tsivgoulis in European Stroke Journal