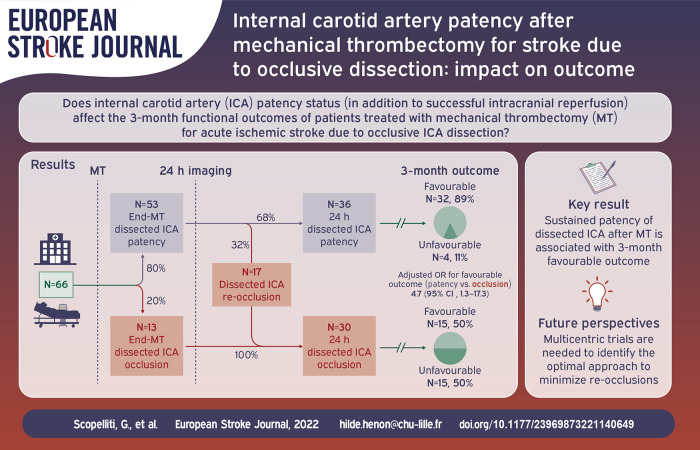
Abstract
Introduction:
Internal carotid artery dissection (ICAD) is a rare cause of acute ischemic stroke with large vessel occlusion (AIS-LVO). We aimed investigating the impact on outcome of internal carotid artery (ICA) patency after mechanical thrombectomy (MT) for AIS-LVO due to occlusive ICAD.
Patients and methods:
We included consecutive patients with AIS-LVO due to occlusive ICAD treated with MT from January 2015 to December 2020 in three European stroke centers. We excluded patients with unsuccessful intracranial reperfusion after MT (modified Thrombolysis in Cerebral Infarction (mTICI) score < 2b). We compared 3-month favorable clinical outcome rate, defined as a modified Rankin scale (mRS) score ⩽2, according to ICA status (patency vs occlusion) at the end of MT and at 24-h follow-up imaging, using univariate and multivariable models.
Results:
Among 70 included patients, ICA was patent in 54/70 (77%) at the end of MT, and in 36/66 (54.5%) patients with 24-h follow-up imaging. Among patients with ICA patency at the end of MT, 32% presented ICA occlusion at 24-h control imaging. Favorable 3-month outcome occurred in 41/54 (76%) patients with ICA patency post-MT and in 9/16 (56%) patients with occluded ICA post-MT (p = 0.21). Rates of favorable outcome were significantly higher in patients with 24-h ICA patency compared to patients with 24-h ICA occlusion (32/36 [89%] vs 15/30 [50%]), with an adjusted odds ratio of 4.67 (95% CI: 1.26–17.25).
Discussion and conclusion:
Obtaining sustained (24-h) ICA patency after MT could be a therapeutic target for improving functional outcome in patients with AIS-LVO due to ICAD.
Keywords: Stroke, large vessel occlusion, mechanical thrombectomy, carotid dissection, internal carotid artery
Graphical abstract.
Introduction
Cervical artery dissection represents up to 10%–25% of all ischemic strokes in patients younger than 50 years, nonetheless it is an uncommon cause of acute ischemic stroke with large vessel occlusion (AIS-LVO).1–4 Although intra-venous thrombolysis (IVT) and mechanical thrombectomy (MT) have been widely proven to be effective in AIS-LVO, patients presenting with ICAD were excluded from randomized controlled trial on MT, 5 leading to uncertainties on the safety and the net clinical benefit of revascularization in patients with ICAD-related strokes.6,7 Previous studies (not focused on reperfusion therapies) showed that occlusion (either isolated or associated with an intracranial downstream occlusion) of the dissected artery complicates about one-third of internal carotid artery dissections (ICAD) and is consistently associated with unfavorable clinical outcome.8,9 Indeed, observational studies showed that MT could be technically difficult in patients with dissected internal carotid artery (ICA), with longer delays to recanalization and potential risk of vessel rupture, dissection extension, or worsening of vessel stenosis.10,11 Therefore, optimal management of ICAD associated with LVO remains controversial, resulting in heterogeneous practices on ICA recanalization, including aggressive treatments such as angioplasty or stenting. 12 Whether or not achieving ICA patency after MT for AIS-LVO due to ICAD has an impact on patient’s clinical outcome is not yet established. The aim of this study was to investigate whether ICA patency after MT has an impact on functional outcome in patients with AIS-LVO due to occlusive ICAD.
Methods
Study population
We performed a retrospective analysis of prospectively collected data from the registries of three European tertiary care centers: Center Hospitalier Universitaire de Lille (Lille, France), Saint Anne Hospital (Paris, France), and Tor Vergata University Hospital (Rome, Italy). Those registries were approved by local institutional ethical review board at each hospital and were designed to collect a standardized dataset of all consecutive patients with acute ischemic stroke treated with MT in their respective district, as described elsewhere.13–15 We included in the study all consecutive patients between January 2015 and December 2020, treated with MT for an AIS-LVO due to extracranial or intracranial ICAD. Patients with non-occlusive ICA dissection were excluded from the study. For the purpose of the study, we also excluded patients with unsuccessful intracranial reperfusion (modified Thrombolysis in Cerebral Infarction (mTICI) score < 2b) after MT.
Clinical and radiological evaluation
We collected data on demographics, vascular risk factors, history of ischemic/hemorrhagic stroke, and previous use of antithrombotic drugs, as previously described.13–16 For all patients, we recorded if intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator was administered before MT. We assessed pre-stroke functional status using modified Rankin Scale (mRS). 17 National Institutes of Health Stroke Scale (NIHSS) score was assessed immediately before MT.
All patients at admission underwent magnetic resonance imaging (MRI) with time-of-flight (TOF) magnetic resonance angiography (MRA), or contrast-enhanced CT-scan with CT-angiography. Pre-MT Alberta Stroke Program Early Computed Tomography Score (ASPECTS) was evaluated on DWI-MRI (when available) or on CT scan. 18 Patients underwent follow-up MRI with contrast-enhanced MRA, or CT scan with CT angiography in case of contraindication, including in both cases cervical vessel imaging control, 24 h after MT or earlier in case of neurological worsening. Senior neuroradiologists from the three centers, blinded to clinical data, analyzed all MRI, CT, and digital subtraction angiography (DSA) images, and assessed pre-MT ASPECTS. We defined the occlusive status of ICA dissection as a complete occlusion or pre-occlusive (⩾90%) stenosis of ICA on the first MT angiograms, as described elsewhere. 19 Site of occlusion was determined on pre-treatment DSA and classified as: (1) isolated intra-/extracranial ICA occlusion (without downstream embolic occlusions) and (2) tandem occlusion (extracranial ICA occlusion + intracranial occlusion).
Acute treatment
Patients presenting with acute ischemic stroke were generally treated according to the European Stroke Organization (ESO) guidelines. 20 Patients were eligible for MT when there was evidence of anterior circulation LVO on TOF/CT angiography sequences, and if it was possible to start MT within 8 h after stroke symptoms recognition. The time window was extended to 24 h from last time known normal after the results of DAWN and DEFUSE-3 trials.21,22 MT was performed by trained neuroradiologists. For all patients, we recorded (1) the time from symptom recognition to MT (for patients with unknown time of stroke symptom onset, the time when the patient was last seen normal was considered) and (2) total MT procedure duration. The procedure was performed under conscious sedation (or under general anesthesia, when necessary), by trans-femoral or radial approach. Angioplasty and artery stenting were generally performed in case of significant residual stenosis after MT or in case of difficult first passage using the catheter alone. Blood pressure during and after mechanical thrombectomy in most cases was managed according to standard international recommendations, suggesting repeated blood pressure measurements and cautious lowering in patients exceeding 220/120 mmHg (in 2018 the limit was lowered to 185/110 in the pre-, intra-, and post-MT)20,23 Patients with ICA stenting received 250 mg of intravenous aspirin, while patients undergoing balloon angioplasty alone did not receive additional antithrombotic treatment during endovascular procedure. All patients received anti-thrombotic treatment, if not contraindicated, after control brain imaging, 24 h after MT procedure; the choice of antithrombotic agents was left to the discretion of the treating physicians.
After MT, all patients were admitted in the stroke unit or intensive care unit. We defined intracranial angiographic success as an mTICI score ⩾ 2b. 24 For the purpose of this study, end-MT ICA status (patency vs occlusion) was evaluated on the last angiogram after MT, and on MRA or CT-angiography imaging at 24-h imaging follow-up 25 ; occlusion in the site of the ICA dissection was defined as a complete (100%) or pre-occlusive (⩾90%) stenosis. 19
Diagnosis of internal carotid artery dissection
The diagnosis of ICAD-related stroke was established by vascular neurologists of the three centers during etiological diagnostic work-up, and it was defined as a radiological evidence of mural hematoma, pseudoaneurysm, long tapering stenosis, intimal flap or double lumen on the internal carotid artery ipsilateral to the stroke lesion.26,27 In patients who underwent brain MRI, diagnosis was based on the evidence of mural hematoma on 3D fat-saturated T1-weighted sequences. 13 Patients with common carotid arteries and iatrogenic dissections (i.e. complications of the endovascular procedure) were not included in the study, as we considered these conditions as distinct clinical entities.
Outcomes
Primary outcome was the favorable functional outcome at 3-month follow-up defined as mRS score ⩽ 2. 17 Modified Rankin Scale score was assessed at 3-month timepoint by experienced vascular neurologists during routine follow-up consultations. When not able to attend the outpatient clinic, the patients (or their caregiver, or treating physicians, or both) were interviewed by telephone to record their vital status and mRS score assessment. Secondary outcomes including overall mRS distribution at 3-month, intracerebral hemorrhages (ICH) assessed on brain MRI/CT scan performed 24 h after MT (classified as symptomatic ICH [sICH], hemorrhagic infarction [HI], and parenchymal hematoma [PH], according to ECASS-3 criteria), 28 decompressive craniectomy after large middle-cerebral artery infarct, 20 and 3-month all-cause mortality.
Statistical analysis
Quantitative variables are expressed as median (interquartile range, IQR) and categorical variables were expressed as frequencies (percentages). Comparisons in outcomes according to ICA status (patency vs occlusion) at end of MT and at 24-h follow-up imaging were done using Fisher’s exact test for binary outcomes and using Mann-Whitney U test for overall mRS distribution at 3-month follow-up. No statistical comparisons were done for outcomes with less than five events. Odds ratios (ORs) and theirs 95% confidence interval (CIs) for patency vs. occlusion were estimated as effect sizes using a penalized logistic regression model with firth’s penalized-likelihood approach to account the small sample size. Comparisons in primary outcome (favorable outcome) were further adjusted for pre-specified confounders (baseline NIHSS score, baseline ASPECTS, IVT, and time from symptoms recognition to MT) using multivariable penalized logistic regression models. Statistical testing was done at the two-tailed α level of 0.05. Data were analyzed using the SAS software package, release 9.4 (SAS Institute, Cary, NC).
Results
Between January 2015 and December 2020, 3737 patients underwent MT for an AIS-LVO in the three participating centers, 104 of whom (2.8%) had an AIS-LVO due ICAD (Figure 1). Nineteen patients were excluded because of a non-occlusive ICA dissection, and 15 because of an unsuccessful intracranial reperfusion (mTICI score 0–2a) after MT. Among 70 included patients, at admission, 22 (31.4%) had an isolated ICA occlusion and 48 (68.6%) had a tandem occlusion. Median age was 49.5 years (interquartile range (IQR) 44–54); 57 (81.4%) were male, and 56 (80.0%) received combined treatment (IVT + MT). The baseline characteristics of the patients included in the study are shown in Table 1.
Figure 1.
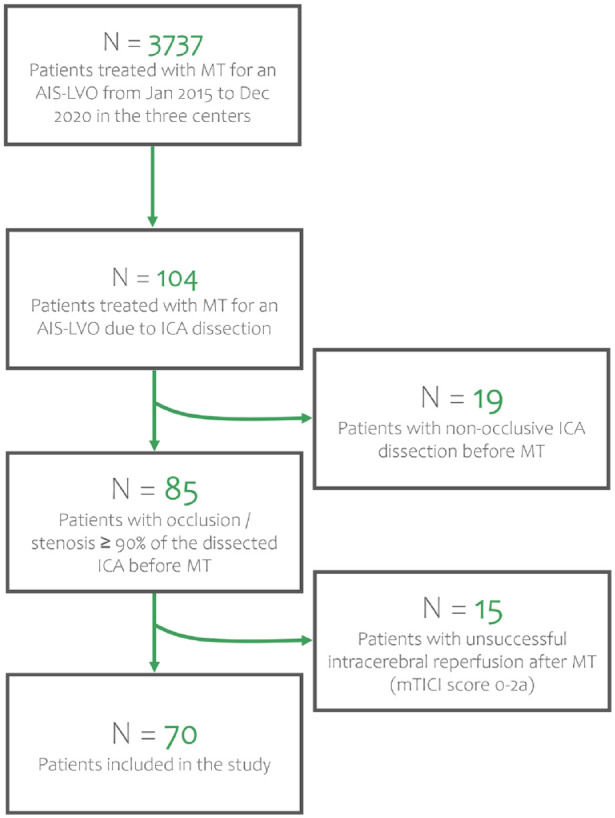
Flowchart of patient inclusion. MT: mechanical thrombectomy; AIS-LVO: acute ischemic stroke due to large vessel occlusion; ICA: internal carotid artery; mTICI: modified Thrombolysis In Cerebral Infarction.
Table 1.
Baseline characteristics of patients, overall and according to dissected internal carotid status (occlusion vs patency) at the end of mechanical thrombectomy.
| All patients | End-MT ICA patency | End-MT ICA occlusion | p-Value | |
|---|---|---|---|---|
| N = 70 | N = 54 | N = 16 | ||
| Age, years | 50 (44–54) | 48 (43–53) | 52 (45–59) | 0.272 |
| Gender (men) | 57 (81.4) | 44 (81.5) | 13 (81.3) | 1.000 |
| Arterial hypertension | 14 (20.0) | 11 (20.4) | 3 (18.8) | 1.000 |
| Diabetes mellitus | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Hypercholesterolemia | 12 (17.1) | 8 (14.8) | 4 (25.0) | 0.450 |
| Smoking | 20 (28.6) | 16 (29.6) | 4 (25.0) | 1.000 |
| Heavy alcohol intake | 4 (5.7) | 2 (3.7) | 2 (12.5) | - |
| History of myocardial infarction | 2 (2.9) | 1 (1.9) | 1 (6.3) | - |
| Previous or current atrial fibrillation | 2 (2.9) | 2 (3.7) | 0 (0.0) | - |
| Previous stroke or TIA | 6 (8.6) | 5 (9.3) | 1 (6.3) | 1.000 |
| mRS before stroke > 1 | 1 (1.4) | 1 (1.9) | 0 (0) | - |
| Anti-thrombotic drugs prior to stroke | ||||
| Anticoagulants | 2 (2.9) | 2 (3.7) | 0 (0) | - |
| Antiplatelets | 8 (11.4) | 7 (13.0) | 1 (6.3) | 0.672 |
| Stroke characteristics | ||||
| NIHSS score before MT | 19 (14–23) | 18 (12–23) | 21 (18–23) | 0.075 |
| DWI MRI/CT scan ASPECT score a | 7 (5–9) | 7 (5–9) | 8 (5–8) | 0.869 |
| Site of occlusion | 0.555 | |||
| Isolated ICA | 22 (31.4) | 16 (29.6) | 6 (37.5) | |
| Tandem | 48 (68.6) | 38 (70.4) | 10 (62.5) | |
| Time from symptom recognition to MT, min | 241 (190–302) | 241 (194–311) | 223 (185–269) | 0.331 |
| Unknown onset time | 12 (17.1) | 10 (18.5) | 2 (12.5) | 0.720 |
| Treatment | ||||
| IVT | 56 (80.0) | 43 (79.6) | 13 (81.3) | 1.000 |
| MT procedure time, min | 46 (34–73) | 46 (34–73) | 45 (39–71) | 0.916 |
| Dissected ICA recanalization technique | ||||
| Only angioplasty | 16 (22.9) | 12 (22.2) | 4 (25.0) | 1.000 |
| Stenting (±angioplasty) | 8 (11.4) | 8 (14.8) | 0 (0.0) | 0.184 |
| None* | 46 (65.7) | 34 (63.0) | 12 (75.0) | 0.550 |
MT: mechanical thrombectomy; TIA: transient ischemic attack; CAD: Carotid artery dissection; mRS: modified Rankin score; DWI: diffusion weighted imaging; MRI: magnetic resonance imaging; ASPECTS: Alberta stroke program early CT score; ICA: internal carotid artery; IVT: intravenous thrombolysis; NIHSS: national institute of health stroke scale; MCA: middle cerebral artery.
Values are expressed as number (%) or median (interquartile range). p-Values were calculated using Mann-Whitney U test for continuous variables and Fisher’s exact test for binary outcomes.
1 missing value.
Passage of the guiding catheter through the occlusion site, without additional angioplasty or stenting.
Clinical and radiological outcomes according to ICA status at the end of MT
Patency of the dissected ICA was observed in 54 patients (77.1%) at the end of MT, while 16 (22.9%) had persistent ICA occlusion on the last MT angiogram. Angioplasty of the dissected ICA was performed in 16 patients (22.9%), artery stenting was performed in eight patients (11.4%); in 46 patients nor angioplasty neither stenting was performed, and in 34 of them (73.9%) ICA patency was obtained simply passing the guiding catheter through the occlusion site, without additional angioplasty or stenting. Patient characteristics according to dissected ICA patency status at the end of MT are shown in Table 1.
Outcomes according to ICA status (patency vs occlusion) at the end of MT are shown on Table 2. Favorable outcome was observed in 41 patients (75.9%) with end-MT ICA patency, and in nine patients (56.3%) with end-MT ICA occlusion, without significant difference (odds ratio (OR) = 2.43; 95% CI, 0.75–7.79; p = 0.21). As shown in Figure 2, when overall 3-month mRS distribution was analyzed, a non-significantly lower mRS was found in patients with end-MT ICA patency by comparison to patients with ICA occlusion at the end of MT (p = 0.058). In a multivariable model adjusted for pre-specified factors (baseline NIHSS score, baseline ASPECTS, IVT, and time from symptoms recognition to MT), ICA patency at the end of MT remained not associated with favorable outcome (adjusted OR, 2.32; 95% CI, 0.64–8.37).
Table 2.
Outcomes of patients according to ICA patency status (patency vs occlusion) at the end of mechanical thrombectomy.
| End-MT ICA patency | End-MT ICA occlusion | OR (95% CI) | p-Value* | |
|---|---|---|---|---|
| N = 54 | N = 16 | |||
| Favorable 3-month functional outcome | 41 (75.9) | 9 (56.3) | 2.43 (0.75–7.79) | 0.21 |
| Death at 3 months | 1 (1.9) | 1 (6.3) | NA | NA |
| Decompressive hemicraniectomy | 5 (9.3) | 1 (6.3) | 1.15 (0.16–8.02) | 1.00 |
| Symptomatic intracerebral hemorrhage | 3 (5.6) | 0 (0) | NA | NA |
| Hemorrhagic transformation | 22 (40.7) | 7 (43.8) | 0.88 (0.28–2.71) | 1.00 |
| HI 1 | 10 (18.5) | 2 (12.5) | - | - |
| HI 2 | 12 (22.2) | 5 (31.3) | - | - |
| Parenchymal hematoma | 5 (9.3) | 1 (6.3) | 1.20 (0.17–8.36) | 1.00 |
| PH 1 | 4 (7.4) | 1 (6.3) | - | - |
| PH 2 | 1 (1.9) | 0 (0) | - | - |
CI, confidence interval; ICA, internal carotid artery; HI, hemorrhagic infarction; OR, odds ratio; NA, not applicable; PH, parenchymal hematoma.
Values are expressed as number (%). We calculated the ORs for patency versus occlusion of ICA using logistic regression model with firth’s penalized-likelihood approach to account the small sample size.
Fisher’s exact test.
Figure 2.
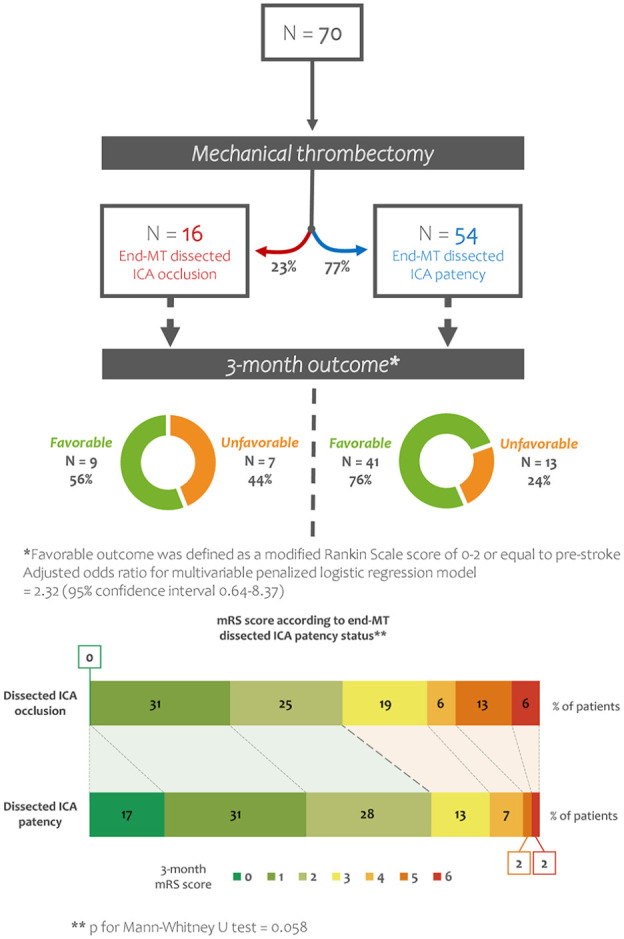
Outcome at 3-month follow-up according to dissected ICA status (patency vs occlusion) at the end of MT. MT: mechanical thrombectomy; ICA: internal carotid artery; mRS: modified Rankin Scale.
Rates of sICH, HI, PH, decompressive craniectomy after large middle-cerebral artery infarct, and 3-month all-cause mortality were not different in patients with and without end-MT ICA patency (Table 2).
Clinical and radiological outcomes according to ICA status 24 h after MT
Of 70 included patients, 66 had both cervical and cerebral vessel follow-up imaging (angio-CT or angio-MRI) 24 h after MT. Of the four patients with missing data on ICA status at 24 h, three had ICA occlusion at the end of MT and one had end-MT ICA patency; none of them died in the first 24 h after MT. Re-occlusion was observed in 17/53 (32.1%) patients with end-MT ICA patency, and all 13 patients with end-MT ICA occlusion had persistent occlusion on 24-h follow-up imaging. Patient characteristics according to dissected ICA patency status at 24-h follow-up imaging are shown in Supplemental Table 1. Patients with ICA patency at 24-h had slightly lower NIHSS scores and higher ASPECTS compared to patients with ICA occlusion at 24-h (both p < 0.05).
Outcomes according to ICA status (patency vs occlusion) at 24-h follow-up imaging after MT are shown on Table 3. Rates of favorable outcomes were significantly higher in patients with ICA patency at 24-h (n = 32/36, 88.9%), compared to patients with 24-h ICA occlusion (n = 15/30, 50.0%; OR = 7.22; 95% CI, 2.11–24.69; p < 0.001). Similarly, ICA patency at 24-h remained significantly associated with a lower likelihood of disability on the overall 3-month mRS analysis (p < 0.001) (Figure 3). The association of ICA patency at 24-h and higher rates of favorable outcome remained significant after pre-specified adjustment (OR = 4.67; 95% CI, 1.26–17.25; p = 0.021).
Table 3.
Outcomes of patients according to ICA patency status (patency vs occlusion) on 24-h follow-up imaging.
| 24-h ICA patency | 24-h ICA occlusion | OR (95% CI) | p-Value* | |
|---|---|---|---|---|
| N = 36 | N = 30 | |||
| Favorable 3-month functional outcome | 32 (88.9) | 15 (50.0) | 7.22 (2.11–24.69) | <0.001 |
| Death at 3 months | 1 (2.8) | 1 (3.3) | NA | NA |
| Decompressive Hemicraniectomy | 3 (8.3) | 3 (10.0) | 0.82 (0.16–4.02) | 1.00 |
| Symptomatic intracerebral hemorrhage | 1 (2.8) | 2 (6.7) | NA | NA |
| Hemorrhagic transformation | 14 (38.9) | 13 (43.3) | 0.84 (0.31–2.24) | 0.80 |
| HI 1 | 7 (19.4) | 4 (13.3) | - | - |
| HI 2 | 7 (19.4) | 9 (30.0) | - | - |
| Parenchymal hematoma | 1 (2.8) | 4 (13.3) | 0.25 (0.03–1.73) | 0.17 |
| PH 1 | 1 (2.8) | 3 (10.0) | - | - |
| PH 2 | 0 (0.0) | 1 (3.3) | - | - |
CI: confidence interval; ICA: internal carotid artery; HI: hemorrhagic infarction; OR: odds ratio; NA: not applicable; PH: parenchymal hematoma.
Values are expressed as number (%). We calculated the ORs for patency versus occlusion of ICA using logistic regression model with firth’s penalized-likelihood approach to account the small sample size.
Fisher’s exact test.
Figure 3.
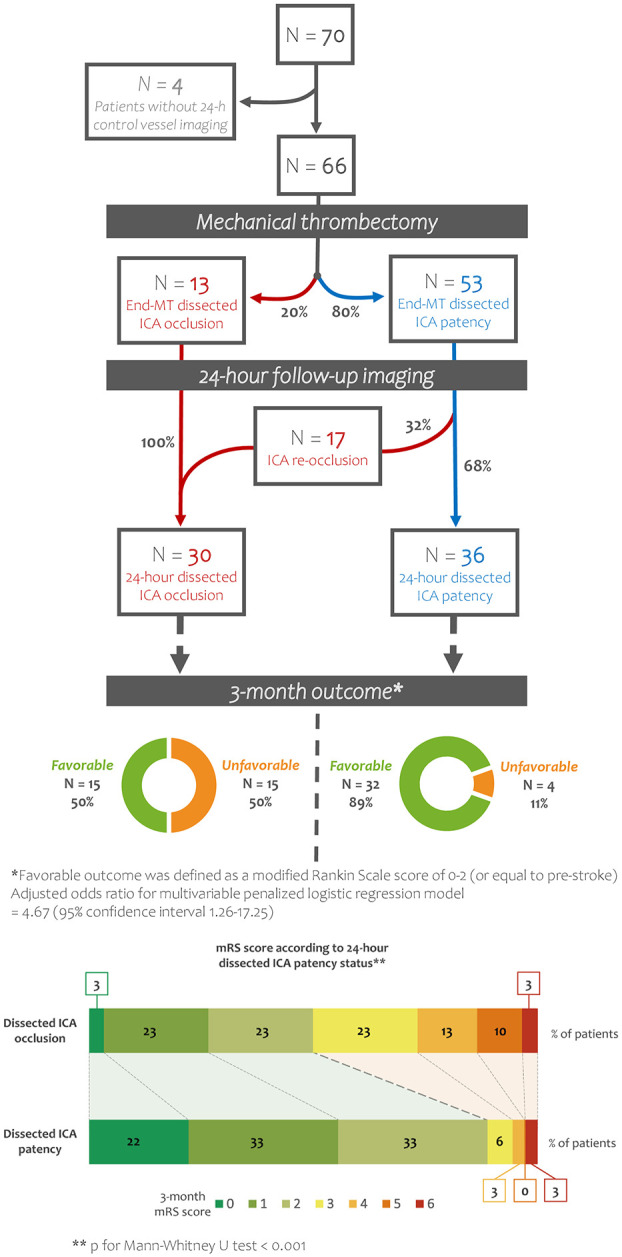
Outcome at 3-month follow-up according to dissected ICA status (patency vs occlusion) at 24-h follow-up imaging after MT. MT, mechanical thrombectomy; ICA, internal carotid artery; mRS, modified Rankin Scale.
Rates of sICH, HI, PH, decompressive craniectomy after large middle-cerebral artery infarct, and 3-month all-cause mortality were not different in patients with and without 24-h ICA patency (Table 3).
Discussion
In this multicentric retrospective analysis on prospectively included patients with AIS-LVO due to ICAD and successful intracranial reperfusion after MT, ICA patency was achieved in nearly 8 out of 10 patients at the end of MT, but with high rates of early (24-h) re-occlusion (one in three patients). ICA status at the end of MT was not significantly associated with 3-month favorable functional outcome, but sustained ICA patency on 24-h imaging follow-up was associated with higher rates of 3-month favorable functional outcome in multivariable analysis (OR = 4.67; 95% CI, 1.26–17.25).
To our knowledge, this is the first study evaluating the potential benefit of achieving ICA patency after MT in patients with AIS-LVO due to ICAD, therefore, the comparison with the literature is limited. In our cohort of patients with AIS-LVO due to ICAD, treated with MT and with successful intracranial reperfusion, we observed a trend toward better functional outcomes at 3-month follow-up in patients with ICA patency compared to patients with ICA occlusion at the end of MT, but this difference did not reach a statistical significance. Despite data were gathered from three large stroke centers, it is likely that the study was underpowered because of our relatively small sample size, that reflects the uncommon occurrence of ICAD as AIS-LVO etiology. Moreover, in one out of three patients with ICA patency at the end of MT, we observed an early ICA re-occlusion on 24-h follow-up imaging. Within the limits of the small sample size, our descriptive data seem not to suggest that baseline characteristics of patients with ICA occlusion due to artery dissection differed in patients with versus without re-occlusion on 24-h follow-up imaging (Supplemental Table 2). We cannot exclude that this high rate of early re-occlusions attenuated the impact of ICA patency status at the end of MT on long-term good functional outcome. Accordingly, in the group of patients with sustained ICA patency on 24-h follow-up imaging, we found significantly higher rates of favorable functional outcome compared to patients with ICA occlusion at 24-h follow-up imaging. Of note, we observed a slight difference in baseline NIHSS and ASPECTS according to 24-h ICA patency status in univariate analyses (median NIHSS of 17 and median ASPECT of 8 in the group of 24 h ICA patency vs median NIHSS of 21 and median ASPECT of 6 in the 24 h ICA occlusion): we cannot exclude that the baseline clinical severity might have driven the decision-making of treating physicians, with more cautious endovascular and medical approaches among more severe patients at higher bleeding risk, that may reflect on the likelihood of achieving persistent patency of the ICA. Nevertheless, we believe that the association between ICA patency at 24-h follow-up and favorable outcomes was not attributable to chance, since the statistical model was still significant after adjusting for baseline NIHSS and ASPECT scores in multivariable model. Accordingly, these results are consistent with those of a previous study on tandem occlusion strokes (mostly of atherosclerotic origin), showing that stable ICA patency at 24-h follow-up imaging is associated with better clinical outcome. 25 Additionally, the rate of favorable outcome of patients with dissected ICA patency at 24-h follow-up was higher than the rate of 3-month functional independence observed in patients aged ⩽50 years with successful intracranial recanalization after MT included in a recent multicenter study. 29 In contrast, only one in two patients with ICA occlusion at 24-h follow-up achieved favorable 3-month outcome despite successful intracranial recanalization, suggesting a specific impact of ICA patency status on 3-month functional outcome.
Possibly, since re-occlusions are frequent, achieving ICA patency at the end of MT is necessary but not sufficient to improve functional outcomes in patients with AIS-LVO due to ICAD. In this perspective, we can suppose that: (i) intra/extra-cranial vessel imaging control 24 h after MT can inform on long-term functional outcome and should be performed systematically; (ii) obtaining ICA patency during MT and minimizing re-occlusion rates after MT through optimization of medical treatment, could both be potential therapeutic targets for improving long-term functional outcome. The ongoing randomized TITAN (Thrombectomy In TANdem lesion) trial, will provide evidence on the potential benefit of combined thrombectomy plus ICA stenting in comparison to thrombectomy alone in patients with acute ischemic stroke due to tandem lesion 30 ; however, further investigations will be required to evaluate the usefulness of ICA stenting or angioplasty in the specific setting of ICAD-related stroke. Interestingly, in our observational cohort, the use of a guiding catheter alone to pass through the dissection site was sufficient to achieve ICA patency at the end of MT in nearly half of patients: whether carotid angioplasty, stenting, or aggressive antithrombotic therapy might improve long-lasting ICA patency is also yet to be established. In our cohort, 16 patients underwent ICA angioplasty, and 8 patients underwent stenting: within the limits of the small number of procedures, we did not observe any clear trend between the use of a specific endovascular technique and the rates of early re-occlusion (Supplemental Table 2). Recently, a pooled analysis from observational registries on 136 patients with tandem lesions due to ICA dissection failed to demonstrate clinical benefit of carotid stenting. 31 The use of IVT has been previously shown to be safe and associated to favorable outcomes in a previous study on tandem lesions due to ICAD 32 ; however, our descriptive analysis (Supplemental Table 2) seems not to suggest that IVT might increase ICA patency rates after MT or reduce early re-occlusions in patients with ICA occlusion due to dissection.
Of note, we found that 50% of patients with persistent ICA occlusion at 24-h follow-up were mRS ⩽ 2 at 3-month follow-up: most likely, the exclusion of patients with unsuccessful intracranial reperfusion and the young median age of included patients could explain the high rate of functional recovery despite ICA occlusion. 13 Moreover, we acknowledge the lack of data on collateral status as a limitation of our study, considering the potential role of good collaterals in providing adequate cerebral perfusion despite ICA occlusion. 33 Concerning radiological outcomes, our data suggest that achieving ICA patency after MT does not increase the risk of intracerebral hemorrhage: this is relevant, since some concerns were raised about hemorrhagic risk related to endovascular treatment in patients with ICAD. 27
The main strengths of this study are the multicentric design and the large number of patients included: ICAD is a rare cause of stroke and represents only a small subset of patients presenting with AIS-LVO (approximately 3% of all patients treated with MT for AIS-LVO in our cohort), making it difficult to build large datasets. Moreover, we only included patients with intracranial successful reperfusion after MT to study the impact of ICA status on outcomes. This study also has several limitations. First, it is a retrospective analysis of prospectively collected data, without randomization to treatment. In this view, achieving ICA patency at the end of the angiography was not the primary objective of the MT performed in our patients (the aim was to achieve intracranial reperfusion); additionally, we did not collect data on patients who were not treated with MT. Moreover, we recognize that the study of clinical-radiological predictors of early re-occlusion after MT would have been of interest; however, this was beyond the aims of our study and hampered by the small number of events (n = 17). We acknowledge the absence of an independent rater for the evaluation of patency status on end-MT angiography and 24-h follow up images; however, considering that our radiological outcome was dichotomized (patent vs occluded), potential interrater disagreements on radiological data interpretation were likely to be limited. We also recognize that the endovascular and medical treatments, namely, the choice of the technique, devices, blood pressure management, and antithrombotic agents was not standardized, and it was tailored on patient’s clinical characteristics, angiographic data, and the neuroradiologists’ experience. The clinical decision-making process was managed locally according to internal protocols. Nevertheless, this study is to be intended as hypothesis generating, and our results showed that the patency status of the dissected ICA has a potential impact on the clinical outcomes after AIS-LVO due to ICAD.
In conclusion, sustained (24-h) ICA patency is associated with 3-month favorable functional outcome after MT in patients with AIS-LVO due to ICAD. Achieving ICA patency at the end of MT is not always sufficient to improve functional outcome, likely because of the high rates of early re-occlusion. Obtaining a sustained (24-h) ICA patency after MT could possibly represent a therapeutic target to improve functional outcome in patients with AIS-LVO due to ICAD. In this perspective, the realization of larger multicentric randomized clinical trials might help identify the optimal endovascular and medical approach to obtain sustained ICA patency after MT.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873221140649 for Internal carotid artery patency after mechanical thrombectomy for stroke due to occlusive dissection: Impact on outcome by Giuseppe Scopelliti, Arnaud Karam, Julien Labreuche, Nicolas Bricout, Federico Marrama, Marina Diomedi, Wagih Ben Hassen, Xavier Leclerc, Charlotte Cordonnier, Hilde Henon and Barbara Casolla in European Stroke Journal
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: All patients were informed that all data acquired during routine clinical practice could be used for research purposes.
Ethical approval: The registries used for this study were approved by local institutional ethical review board at each hospital and were designed to collect a standardized dataset of all consecutive patients with acute ischemic stroke treated with MT. According to the French legislation, the approval for data pooling was not required because this study only implied retrospective analysis of anonymized data collected as part of routine care.
Guarantor: HH.
Author contributions: GS designed and conceptualized the study, analyzed, and interpreted all data, and drafted the manuscript. JL, AK, BC, and HH designed and conceptualized the study, analyzed, and interpreted all data, drafted, and reviewed the manuscript. NB, XL, CC, WB, FM, and MD interpreted data and reviewed the manuscript.
ORCID iDs: Giuseppe Scopelliti  https://orcid.org/0000-0002-2313-2124
https://orcid.org/0000-0002-2313-2124
Barbara Casolla  https://orcid.org/0000-0003-4199-995X
https://orcid.org/0000-0003-4199-995X
Supplemental material: Supplemental material for this article is available online.
References
- 1. Engelter ST, Rutgers MP, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke 2009; 40: 3772–3776. [DOI] [PubMed] [Google Scholar]
- 2. Leys D, Bandu L, Hénon H, et al. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology 2002; 59: 26–33. [DOI] [PubMed] [Google Scholar]
- 3. Yesilot Barlas N, Putaala J, Waje-Andreassen U, et al. Etiology of first-ever ischaemic stroke in European young adults: the 15 cities young stroke study. Eur J Neurol 2013; 20: 1439. [DOI] [PubMed] [Google Scholar]
- 4. Schlemm L, von Rennenberg R, Siebert E, et al. Mechanical thrombectomy in patients with cervical artery dissection and stroke in the anterior or posterior circulation - a multicenter analysis from the German Stroke Registry. Neurol Res Pract 2021; 3: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. New Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 6. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin J, Sun Y, Zhao S, et al. Safety and efficacy of thrombolysis in cervical artery dissection-related ischemic stroke: a meta-analysis of observational studies. Cerebrovasc Dis 2016; 42: 272–279. [DOI] [PubMed] [Google Scholar]
- 8. Traenka C, Grond-Ginsbach C, Goeggel Simonetti B, et al. Artery occlusion independently predicts unfavorable outcome in cervical artery dissection. Neurology 2020; 94: e170–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benninger DH, Georgiadis D, Kremer C, et al. Mechanism of ischemic infarct in spontaneous carotid dissection. Stroke 2004; 35: 482–485. [DOI] [PubMed] [Google Scholar]
- 10. Kurre W, Bansemir K, Aguilar Pérez M, et al. Endovascular treatment of acute internal carotid artery dissections: technical considerations, clinical and angiographic outcome. Neuroradiol 2016; 58: 1167–1179. [DOI] [PubMed] [Google Scholar]
- 11. Xianjun H, Zhiming Z. A systematic review of endovascular management of internal carotid artery dissections. Interv Neurol 2013; 1: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke A. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 13. Karam A, Bricout N, Khyeng M, et al. Safety and outcome of mechanical thrombectomy in ischaemic stroke related to carotid artery dissection. J Neurol 2022; 269: 772–779. [DOI] [PubMed] [Google Scholar]
- 14. Ben Hassen W, Touloupas C, Benzakoun J, et al. Impact of repeated clot retrieval attempts on infarct growth and outcome after ischemic stroke. Neurology 2021; 97: e444–e453. [DOI] [PubMed] [Google Scholar]
- 15. Sallustio F, Motta C, Merolla S, et al. Heparin during endovascular stroke treatment seems safe. J Neuroradiol 2019; 46: 373–377. [DOI] [PubMed] [Google Scholar]
- 16. Ducroquet A, Leys D, Al Saabi A, et al. Influence of chronic ethanol consumption on the neurological severity in patients with acute cerebral ischemia. Stroke 2013; 44: 2324–2326. [DOI] [PubMed] [Google Scholar]
- 17. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 18. Barber PA, Hill MD, Eliasziw M, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry 2005; 76: 1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papanagiotou P, Haussen DC, Turjman F, et al. Carotid stenting with antithrombotic agents and intracranial thrombectomy leads to the highest recanalization rate in patients with acute stroke with tandem lesions. JACC Cardiovasc Interv 2018; 11: 1290–1299. [DOI] [PubMed] [Google Scholar]
- 20. Ringleb PA, Bousser MG, Ford G, et al. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25: 457–507. [DOI] [PubMed] [Google Scholar]
- 21. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 23. Ahmed N, Steiner T, Caso V, et al. Recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 13–15 November 2016. Eur Stroke J 2017; 2: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke 2018; 13:612–632. [DOI] [PubMed] [Google Scholar]
- 25. Bricout N, Personnic T, Ferrigno M, et al. Day 1 extracranial internal carotid artery patency is associated with good outcome after mechanical thrombectomy for tandem occlusion. Stroke 2018; 49: 2520–2522. [DOI] [PubMed] [Google Scholar]
- 26. Compter A, Schilling S, Vaineau CJ, et al. Determinants and outcome of multiple and early recurrent cervical artery dissections. Neurology 2018; 91: e769–e780. [DOI] [PubMed] [Google Scholar]
- 27. Debette S, Mazighi M, Bijlenga P, et al. ESO guideline for the management of extracranial and intracranial artery dissection. Eur Stroke J 2021; 6: XXXIX–LXXXVIII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 29. Yeo LLL, Chen VHE, Leow AST, et al. Outcomes in young adults with acute ischemic stroke undergoing endovascular thrombectomy: a real-world multicenter experience. Eur J Neurol 2021; 28: 2736–2744. [DOI] [PubMed] [Google Scholar]
- 30. Zhu F, Hossu G, Soudant M, et al. Effect of emergent carotid stenting during endovascular therapy for acute anterior circulation stroke patients with tandem occlusion: a multicenter, randomized, clinical trial (TITAN) protocol. Int J Stroke 2021; 16: 342–348. [DOI] [PubMed] [Google Scholar]
- 31. Marnat G, Lapergue B, Sibon I, et al. Safety and outcome of carotid dissection stenting during the treatment of tandem occlusions: a pooled analysis of TITAN and ETIS. Stroke 2020; 51: 3713–3718. [DOI] [PubMed] [Google Scholar]
- 32. Marnat G, Sibon I, Bourcier R, et al. Thrombolysis improves reperfusion and the clinical outcome in tandem occlusion stroke related to cervical dissection: TITAN and ETIS pooled analysis. J Stroke 2021; 23: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamauchi H, Kudoh T, Sugimoto K, et al. Pattern of collaterals, type of infarcts, and haemodynamic impairment in carotid artery occlusion. J Neurol Neurosurg Psychiatry 2004; 75: 1697–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873221140649 for Internal carotid artery patency after mechanical thrombectomy for stroke due to occlusive dissection: Impact on outcome by Giuseppe Scopelliti, Arnaud Karam, Julien Labreuche, Nicolas Bricout, Federico Marrama, Marina Diomedi, Wagih Ben Hassen, Xavier Leclerc, Charlotte Cordonnier, Hilde Henon and Barbara Casolla in European Stroke Journal