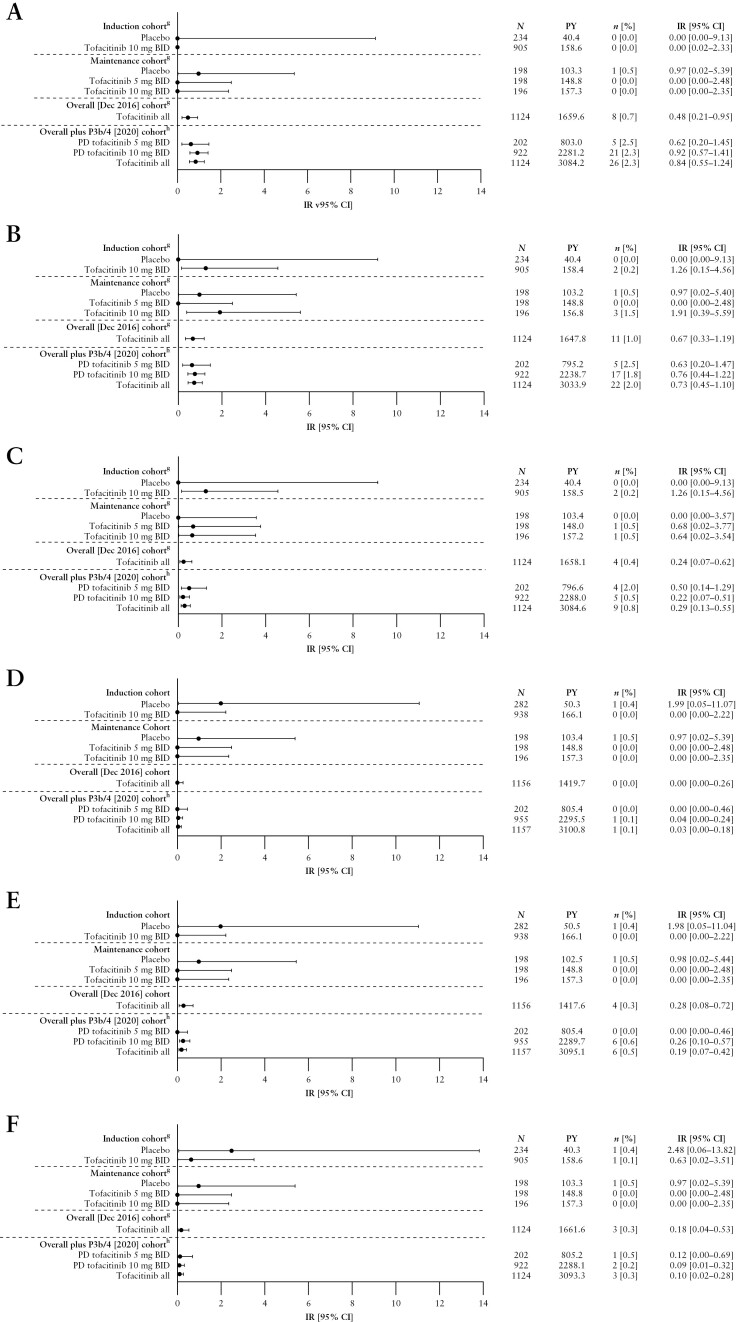
Figure 2.
Incidence of [A] malignancies [excluding NMSC],a,b,c [B] NMSC,a,b,c [C] MACE,a,b,c [D] DVT,d,e [E] PEd,e and [F] GI perforations,b,c,d,f by cohort.
aFor the Maintenance Cohort, events that occurred >28 days after the last dose of the study drug were excluded; for the Overall [Dec 2016] and Overall plus P3b/4 [2020] Cohort, all events, including those outside the 28-day risk period, were included.
bAdjudicated events.
cFor the Overall plus P3b/4 [2020] Cohort, N = 922 and N = 1124 for the PD tofacitinib 10 mg BID and tofacitinib all groups, respectively [excludes phase 2 study data, as the phase 2 induction study took place prior to the establishment of the adjudication committees].
dEvents that occurred >28 days after the last dose of the study drug were excluded.
eVTEs included adjudicated events from OCTAVE Open and RIVETING plus selected events from phase 2/3 studies from the Narrow Standardized MedDRA query for embolic and thrombotic events.
fGI perforation excludes preferred terms of pilonidal cyst, perirectal abscess, rectal abscess, anal abscess, perineal abscess and any preferred terms containing the term fistula.
gIncludes data previously reported by Sandborn et al.7; the previously reported Overall [Dec 2016] Cohort included data from OCTAVE Open up to December 2016 [≤4.4 years of exposure].
hThe Overall plus P3b/4 [2020] Cohort includes final data from OCTAVE Open [final data cut-off: August 24, 2020], and data from RIVETING [interim data cut-off: February 20, 2020; ≤7.8 years of exposure].
AE, adverse event; BID, twice daily; CI, confidence interval; DVT, deep vein thrombosis; GI, gastrointestinal; IR, incidence rate [unique patients with events/100 PY of exposure]; MACE, major adverse cardiovascular events; N, number of patients treated in the treatment group; n, number of unique patients with a particular AE; MedDRA, Medical Dictionary for Regulatory Activities; NMSC, non-melanoma skin cancer; PD, predominant dose; PE, pulmonary embolism; PY, patient-years; VTE, venous thromboembolic event.