Figure 1. A combination of axonal translation of Pak5 mRNA and AKT activation reprograms mitochondrial motility in injured axons.
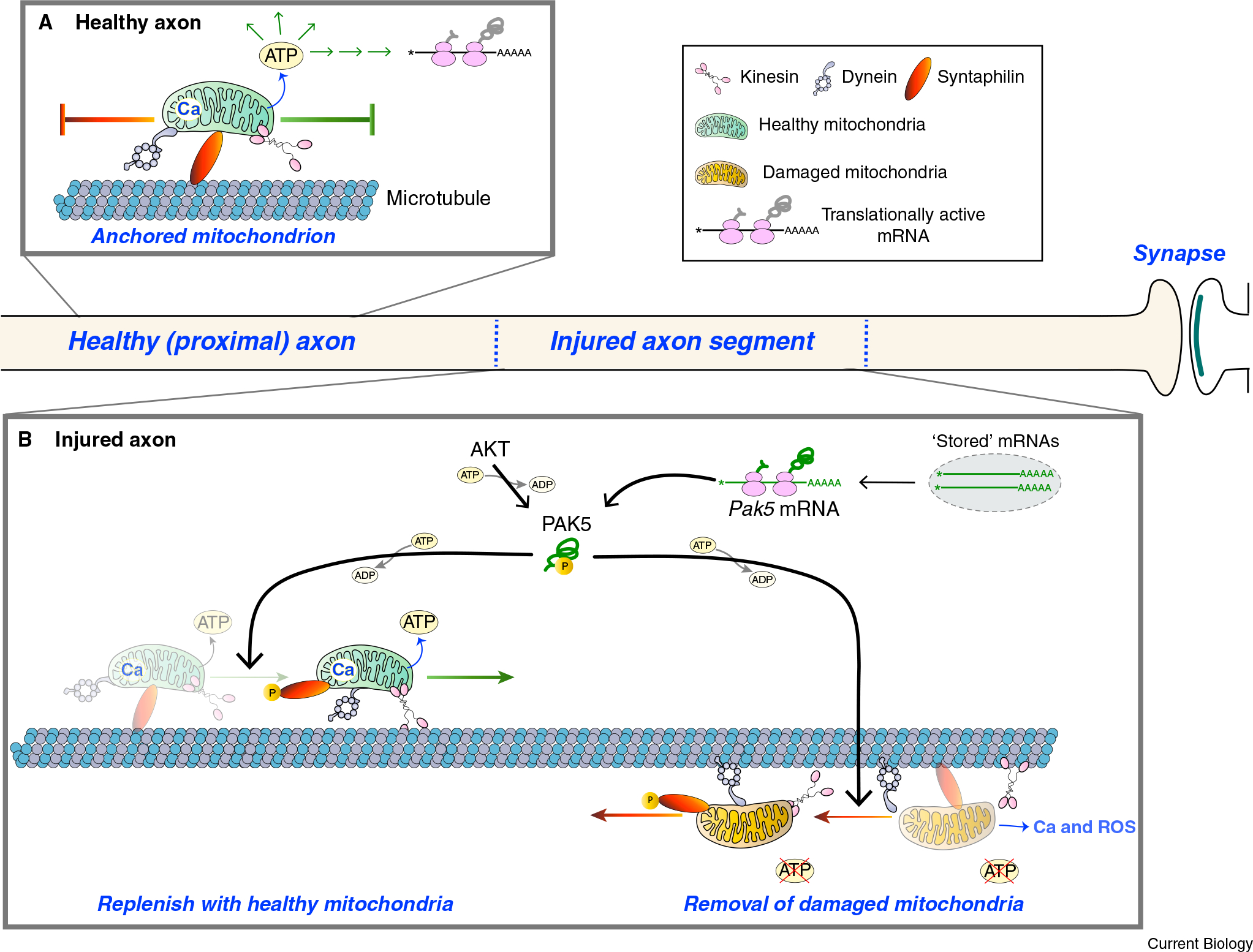
(A) In a mature uninjured axon, most mitochondria are anchored in place on microtubules by SNPH. This provides a localized source of energy (ATP) and Ca2+ (Ca) storage. (B) Axonal injury damages mitochondria by reducing oxidative phosphorylation so that the local source of ATP is at risk. This also results in the release of Ca2+ from mitochondria and a localized increase in reactive oxygen species (ROS). The Miro–Milton complex (not shown) provides an adapter for the association of mitochondria with kinesin and dynein motor proteins, and release of Ca2+ from mitochondria into the axoplasm would further impact mitochondrial motility by promoting the dissociation of Miro from Milton. Huang et al.2 show that injury can trigger the translation of Pak5 mRNA, with AKT activating the nascent PAK5 protein, and activated PAK5 phosphorylating SNPH to release the SNPH mitochondrial anchor. This brings to the injury site functional mitochondria that promote both neuroprotection and axon regeneration, as well as removing damaged mitochondria that can impede neuroprotection and neural repair.
