Summary
Background
The diagnosis of symptomatic Alzheimer's disease is a clinical challenge in adults with Down syndrome. Blood biomarkers would be of particular clinical importance in this population. The astrocytic Glial Fibrillary Acidic Protein (GFAP) is a marker of astrogliosis associated with amyloid pathology, but its longitudinal changes, association with other biomarkers and cognitive performance have not been studied in individuals with Down syndrome.
Methods
We performed a three-centre study of adults with Down syndrome, autosomal dominant Alzheimer's disease and euploid individuals enrolled in Hospital Sant Pau, Barcelona (Spain), Hospital Clinic, Barcelona (Spain) and Ludwig-Maximilians-Universität, Munich (Germany). Cerebrospinal fluid (CSF) and plasma GFAP concentrations were quantified using Simoa. A subset of participants had PET 18F-fluorodeoxyglucose, amyloid tracers and MRI measurements.
Findings
This study included 997 individuals, 585 participants with Down syndrome, 61 Familial Alzheimer's disease mutation carriers and 351 euploid individuals along the Alzheimer's disease continuum, recruited between November 2008 and May 2022. Participants with Down syndrome were clinically classified at baseline as asymptomatic, prodromal Alzheimer's disease and Alzheimer's disease dementia. Plasma GFAP levels were significantly increased in prodromal and Alzheimer's disease dementia compared to asymptomatic individuals and increased in parallel to CSF Aβ changes, ten years prior to amyloid PET positivity. Plasma GFAP presented the highest diagnostic performance to discriminate symptomatic from asymptomatic groups (AUC = 0.93, 95% CI 0.9−0.95) and its concentrations were significantly higher in progressors vs non-progressors (p < 0.001), showing an increase of 19.8% (11.8–33.0) per year in participants with dementia. Finally, plasma GFAP levels were highly correlated with cortical thinning and brain amyloid pathology.
Interpretation
Our findings support the utility of plasma GFAP as a biomarker of Alzheimer's disease in adults with Down syndrome, with possible applications in clinical practice and clinical trials.
Funding
AC Immune, La Caixa Foundation, Instituto de Salud Carlos III, National Institute on Aging, Wellcome Trust, Jérôme Lejeune Foundation, Medical Research Council, Alzheimer's Association, National Institute for Health Research, EU Joint Programme–Neurodegenerative Disease Research, Alzheimer's Society, Deutsche Forschungsgemeinschaft, Stiftung für die Erforschung von Verhaltens, Fundación Tatiana Pérez de Guzmán el Bueno & European Union's Horizon 2020 und Umwelteinflüssen auf die menschliche Gesundheit.
Keywords: GFAP, Plasma, Down syndrome, Alzheimer's disease
Research in context.
Evidence before this study
We searched previous literature using PubMed, meeting abstracts and presentations. We searched PubMed on January 18th, 2023, for published studies with the terms “glial fibrillary acidic protein” OR “GFAP” AND “Down syndrome” AND “plasma” AND “CSF” and the search retrieved zero results. When CSF was deleted from the search code, two publications were retrieved. The first study measured the levels of plasma biomarkers in 90 participants with Down syndrome. The reported data are the baseline measurements of a longitudinal study that started in 2019 (paused by the COVID pandemic) and aims to follow the participants for 32 months. The second publication is a cross-sectional, multicentre study with 300 Down syndrome participants and a control group of 37 non-DS siblings, in which they investigate plasma biomarker combinations to detect tau pathological changes in Downs syndrome. Plasma GFAP and p-tau217—but no other plasma biomarkers—were consistently associated with abnormal tau-PET and Aβ-PET status in models covaried for age.
Added value of this study
This longitudinal study assesses the potential of plasma and CSF GFAP as a diagnostic and prognostic biomarker for Alzheimer's disease in Down syndrome and compares the dynamics of this biomarker with both autosomal dominant and sporadic Alzheimer's disease. First, we show that plasma GFAP is the biomarker that changes most along the Alzheimer's disease continuum, with increases from very early preclinical Alzheimer's disease and continues to raise in the dementia stage. Second, we compare the evolution in Down syndrome and autosomal dominant Alzheimer's disease. The two genetically determined forms of the disease present the same temporality, with changes that start in parallel to the decreases in CSF Aβ levels more than 25 years before symptom onset and 10 years prior to increases in amyloid PET uptake. Third, we show that the diagnostic performance of plasma GFAP was the highest among the studied biomarkers to discriminate symptomatic from asymptomatic Alzheimer's disease individuals. Fourth, we determine that plasma GFAP levels can be used to predict disease progression and cognitive decline and show that longitudinal trajectories of plasma GFAP increase during the dementia stage. Finally, we establish that plasma GFAP levels are associated with amyloid brain pathology as measured by Aβ PET.
Implications of all the available evidence
Clinical diagnosis of symptomatic Alzheimer's disease is complex in adults with Down syndrome, due to pre-existing and varying intellectual disability, which may overshadow the Alzheimer's disease-related cognitive decline. Cost-effective and easily accessible biomarkers would greatly assist in diagnosing and monitoring Alzheimer's disease in this population. The high diagnostic and prognostics performance of plasma GFAP to diagnose Alzheimer's disease in people with Down syndrome indicates that this biomarker could be of great utility in clinical practice and as a screening tool in clinical trials.
Introduction
The lifetime risk of Alzheimer's disease in people with Down syndrome is higher than 95% by the seventh decade of life, and is the leading cause of death in this population.1 The strong association between Down syndrome and Alzheimer's disease is attributed to the triplication of the amyloid precursor protein (APP) encoded on chromosome 21.2 Overexpression of APP leads to overproduction of amyloid-β (Aβ) peptide and increased deposition in the brain, similarly to autosomal dominant forms of the disease.3 Therefore, Down syndrome is considered as a form of genetically determined Alzheimer's disease.4
Clinical diagnosis of prodromal and Alzheimer's disease dementia in people with Down syndrome is a diagnostic challenge. The change in cognitive functioning4 can be difficult to determine given the variable levels of intellectual disability associated with the syndrome,5 and to the lack of recorded baseline cognitive performance prior to symptom onset. Reliable biological markers would be of clinical importance in this population. Cerebrospinal fluid (CSF) biomarkers Aβ42/Aβ40, phosphorylated tau (p-tau) and neurofilament light chain (NfL), which reflect Aβ deposition, neurofibrillary tangle pathology and neurodegeneration, have excellent diagnostic performances.6,7 However, the need of a lumbar puncture limits their use in routine clinical practice.
Validated blood-based biomarkers have clear advantages due to their accessibility and cost-effectiveness. Recent advances in ultra-sensitive detection methods have allowed for the quantification of low abundant proteins reflecting brain pathological changes in blood: p-tau,8, 9, 10 NfL11,12 and Glial Fibrillary Acidic Protein (GFAP).13, 14, 15, 16, 17, 18 In Alzheimer's disease in Down syndrome, plasma p-tau181 differentiates asymptomatic individuals from those with dementia,19,20 and plasma p-tau217 accurately identifies both tau and Aβ pathologies determined by PET imaging.21 Plasma NfL levels correlate with age and cognitive decline and have excellent diagnostic and prognostic performances.3,12,22 Finally, plasma GFAP has been shown to be increased in very early stages of the disease, when individuals are still Aβ-PET negative, and consistently associate with abnormal tau- and Aβ-PET.23 However, longitudinal studies assessing CSF and plasma GFAP levels in individuals with Down syndrome are missing.
In this work, we measured plasma and CSF GFAP concentrations in adults with Down syndrome, in comparison to sporadic and autosomal dominant and sporadic Alzheimer's disease. We aimed to i) describe the changes of plasma and CSF GFAP levels along the Alzheimer's disease continuum and with age; ii) determine the diagnostic and prognostic performance of GFAP; iii) describe longitudinal trajectories of GFAP levels in the different stages of the Alzheimer's disease continuum; and iv) establish associations with fluid (plasma and CSF Aβ, p-tau181 and NfL) and neuroimaging biomarkers (cortical thinning, glucose hypometabolism and Aβ PET).
Methods
Study design and participants
We performed a three-centre longitudinal cohort study of adults with Down syndrome, autosomal dominant Alzheimer disease and euploid individuals along the Alzheimer's disease continuum in Hospital of Sant Pau, Barcelona (Spain), Hospital Clínic, Barcelona (Spain), and Munich (Germany). Adults with Down syndrome in Barcelona were recruited from a population-based health plan designed to screen for Alzheimer's disease dementia, which includes yearly neurological and neuropsychological assessments. Those subjects interested in research studies are included in the Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) cohort,3,7 and the Alzheimer-21 cohort in Munich. We also recruited euploid controls and sporadic Alzheimer's disease patients from the Sant Pau Initiative on Neurodegeneration (SPIN cohort). Finally, we included a third cohort of autosomal dominant Alzheimer's disease mutation carriers that were evaluated at Hospital Clinic (Barcelona, Spain). The period of recruitment was November 2008–May 2022.
Ethics
All procedures in this study were approved by the Sant Pau Ethics Committee (IIBSP-NGF-2018-36 and IIBSP-DOW-2014-30), the ethics committee of Ludwig-Maximilians-University Munich and the Hospital Clinic Ethics Committee, following the standards for medical research in humans recommended by the Declaration of Helsinki. All participants or their legally authorized representative gave written informed consent before enrollment. We included all adults with Down syndrome that had plasma or CSF samples available.
Procedures
We administered a semi-structured adapted health questionnaire to the caregivers, the Cambridge Examination for Mental Disorders of Older People with Down syndrome and others with intellectual disabilities (CAMDEX-DS) developed in Cambridge, and also adapted to the Spanish24 and German25 population. The information was obtained through family interview and review of medical or educational record for past assessment results. We classified Down syndrome participants into asymptomatic, prodromal Alzheimer's disease or Alzheimer's disease dementia in a consensus meeting between the neurologist/psychiatrist and the neuropsychologists who assessed them, blind to the biomarker data, as previously described.3,7 Participants classified as uncertain (n = 41) were excluded from the study. Euploid participants underwent a structured neurological assessment and a comprehensive neuropsychological battery. For the prognostic evaluation, participants were subsequently classified as “progressors” when there was a change in the clinical diagnosis along the Alzheimer's disease continuum (from asymptomatic to prodromal or dementia and from prodromal to dementia). Participants that remained in the same Alzheimer's disease diagnostic category at the end of follow-up and for at least 2 years after the initial evaluation were classified as “non-progressors” (Supplementary Methods). Genetic screening of trisomy 21 was assessed in adults with Down syndrome and APOEε4 carrier status was obtained following previously published protocols.3,7
CSF and blood samples were acquired concurrently following established procedures. Plasma and CSF GFAP, NfL and p-tau181 concentrations were measured using Single Molecule Array (Simoa) and CSF YLK-40 by ELISA (Supplementary Methods). A subset of participants underwent a basal 3T-MRI (n = 194), 18F-Fluorodeoxyglucose (FDG) PET (n = 92) and either 18F-Florbetapir or 18F-Flutemetamol PET acquisitions (n = 75) as previously described3 (Supplementary Methods).
Statistical analysis
All the statistical analyses were performed using R statistical software version 3.6.3. Baseline characteristics were summarized using standard descriptive statistics. Continuous variables were described as median [IQR] and categorical data were summarized as absolute frequencies and percentages. Data were log transformed to normalize distribution when needed. All significance tests were two-sided with the statistical significance set at 5%.
For each biomarker, we calculated fold-change relative to median levels of their respective asymptomatic groups. Fold-changes were compared through age-adjusted ANCOVA. To determine the temporality of plasma biomarkers changes we fitted a first degree locally estimated scatterplot smoothing curve in controls, adults with Down syndrome and mutation carriers independently.
The diagnostic performance of the different biomarkers was assessed with receiver operating characteristic (ROC) curve analyses. To compare the different areas under the curve (AUC) the DeLong's test was used. Bonferroni correction was applied for multiple comparisons. Longitudinal changes in plasma GFAP levels in the Down syndrome population and their association with clinical progression status were assessed through linear mixed models.
Check Supplementary Material for additional information on the specific statistical test used for each analysis.
Role of the funders
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
This study included 997 individuals, 585 participants with Down syndrome, 61 autosomal dominant Alzheimer's disease mutation carriers and 351 euploid participants, recruited between November 2008 and May 2022. Table 1 shows the demographics, cognitive and plasma biomarkers across groups of all participants included in the analyses, and Supplementary Table S1 includes the information of participants with CSF samples available. Plasma and CSF biomarkers mean concentrations are represented in Supplementary Figure S1. Demographics, cognitive scores, and plasma concentrations did not differ between the subgroup of participants with CSF and the overall sample. As expected, participants with Down syndrome asymptomatic for Alzheimer's disease were significantly younger than those in the prodromal Alzheimer's disease (estimated difference of 13.2 years; 95% CI 10.8–15.7; p < 0.001) and Alzheimer's disease dementia groups (estimated difference of 14.9 years; 95% CI 13.0–16.8; p < 0.001). Likewise, asymptomatic mutation carriers were younger than symptomatic (estimated difference of 13.6 years; 95% CI 8.7–18.4; p < 0.001); and in the euploid group, cognitively normal individuals were significantly younger than prodromal (estimated difference of 21.1 years; 95% CI 18.8–23.5; p < 0.001) and Alzheimer's disease dementia patients (estimated difference of 19.9 years; 95% CI 17.2–22.7; p < 0.001).
Table 1.
Demographics, cognitive and plasma biomarkers across groups of all participants included in the analyses.
| aDS | pDS | dDS | CN | MCI-AD | AD | aMC | sMC | |
|---|---|---|---|---|---|---|---|---|
| Participant samples (N) | 387 | 69 | 129 | 198 | 92 | 61 | 26 | 35 |
| Age (years)—mean (SD) | 37.8 (10.2) | 51 (5.3) | 52.7 (5.64) | 50.4 (12.6) | 71.5 (6.66) | 70.3 (7.74) | 35.2 (8.78) | 48.8 (10) |
| Sex | ||||||||
| Female/male (N) | 171/216 | 32/37 | 62/67 | 136/62 | 53/39 | 35/26 | 21/5 | 22/13 |
| Female/male (%) | 44.2/55.8 | 46.4/53.6 | 48.1/51.9 | 68.7/31.3 | 57.6/42.4 | 57.4/42.6 | 80.8/19.2 | 62.9/37.1 |
| APOE4 status | ||||||||
| APOE4−/APOE4+ (N) | 283/69 | 45/20 | 90/26 | 150/48 | 33/57 | 28/33 | 20/3 | 30/4 |
| APOE4−/APOE4+ (%) | 80.4/19.6 | 69.2/30.8 | 77.6/22.4 | 75.8/24.2 | 36.7/63.3 | 45.9/54.1 | 87/13 | 88.2/11.8 |
| Follow-up time (years)—mean (SD) | 3.33 (2.52) | 2.72 (2.36) | 2.07 (1.85) | 2.28 (2.29) | 2.56 (2.43) | 2.61 (2.37) | NA | NA |
| Number of visits—mean (SD) | 5.96 (3.45) | 7.57 (5.06) | 6.24 (4.51) | 2.49 (1.56) | 4.03 (3.05) | 4.39 (3.69) | NA | NA |
| Plasma GFAP (pg/ml)—mean (SD) | 111 (72.8) | 280 (151) | 358 (217) | 82.7 (43.3) | 232 (94.9) | 285 (128) | 111 (72.8) | 280 (151) |
| Plasma NFL (pg/ml)—mean (SD) | 11.7 (8.07) | 23.7 (15.2) | 31.3 (18) | 7.47 (4.35) | 16.2 (7.46) | 17.4 (7.33) | 7.32 (3.9) | 19 (12.3) |
| Plasma pTau181 (pg/ml)—mean (SD) | 13.1 (9.94) | 25.5 (10.9) | 29.9 (14.1) | 13.3 (13.7) | 22.2 (10.2) | 22 (9.7) | 14.1 (9.53) | 28.4 (17.2) |
Asymptomatic Down syndrome (aDS), prodromal Alzheimer's disease (pDS) and Alzheimer's disease dementia (dDS); Cognitively Normal (CN), Mild Cognitively Impaired Alzheimer's disease (MCI-AD) and Alzheimer's disease dementia (AD); Familial Alzheimer's disease mutation carriers were grouped into asymptomatic (aMC) and symptomatic (sMC).
GFAP was the plasma biomarker with the highest fold-change in dementia participants compared with the asymptomatic group (3.4-fold-change in Down syndrome and euploid controls, and 3 times in symptomatic mutation carriers compared to asymptomatic carriers) (Supplementary Figure S2, Supplementary Table S2). Contrarily to plasma, CSF GFAP was the biomarker with the lowest fold-change between asymptomatic and dementia groups, 2.2-fold change in Down syndrome and 2-fold change in euploid controls. Fig. 1a represents the changes in plasma GFAP levels along life span and related to amyloid biomarker changes in the three populations. In Down syndrome, plasma GFAP starts to increase in the mid-late 20s, close to CSF Aβ decreases, and approximately 10 years before the increases in amyloid PET uptake. In contrast, plasma p-tau181 starts to increase in the mid-30s more closely related with the start in the elevations of amyloid PET (Fig. 1b). Importantly, the same temporality was observed in autosomal dominant Alzheimer's disease.
Fig. 1.
Plasma GFAP (a) and pTau181 (b) changes across age in Down syndrome, euploid controls and autosomal dominant Alzheimer’s disease mutation carriers. Values for Down syndrome individuals are represented in red, for euploid controls in blue and for autosomal dominant Alzheimer's disease mutation carriers in orange. The central lines indicate the fitted LOESS model for each group and the shadowed ribbons show the 95% confidence level intervals. Estimated years to symptoms onset (EYO) are calculated as the difference between each participant's age and the mean age at symptoms onset in each mutation in autosomal dominant Alzheimer's disease. For Down syndrome participants mean age of symptoms onset is set at 53.8 years. For comparison purposes, the same is used as a reference in euploid controls.
We used ROC analyses to evaluate the diagnostic performance of the different plasma and CSF biomarkers in asymptomatic vs symptomatic Down syndrome, autosomal dominant and sporadic Alzheimer's disease (Supplementary Figure S3). In all three populations, plasma GFAP, NfL and p-tau181 showed very high accuracy, and in the case of Down syndrome, plasma GFAP had an AUC = 0.93, 95% CI 0.9−0.95. This value was not significantly superior to that of plasma NfL or p-tau181 (DeLong test adjusted p-value = 0.53 and p = 0.49, respectively). However, the combination of plasma GFAP with age, sex and the APOE4 haplotype resulted in a more accurate model (AUC = 0.95, 95% CI 0.93−0.97), and this value was significantly higher than that of age, sex and APOE4 (AUC = 0.91; deLong test adjusted p-value = 0.00046), indicating that plasma GFAP provides additional value to the model. This was also the case when age was restricted to participants older than 40 years (from AUC = 0.80 to 0.89, DeLong's test adjusted p-value = 0.00026) (data not shown). In addition, sensitivity analysis of the accuracy of plasma biomarkers along the age-span of participants with Down syndrome showed that plasma GFAP had acceptable accuracy (AUC > 0.80) to discriminate symptomatic from asymptomatic participants until later ages compared to NfL, pTau181 or age (Supplementary Figure S4). In contrast, CSF GFAP had the lowest performance in discriminating asymptomatic from symptomatic individuals (AUC = 0.84, 95% CI 0.78−0.89). When ROC analyses were performed, comparing asymptomatic vs prodromal and asymptomatic vs dementia participants (Supplementary Figures S5 and S6) we obtained qualitatively similar results. Cut-off values that yielded maximum Youden indices to discriminate between asymptomatic and symptomatic participants were similar in all three populations but were closer between Down syndrome and autosomal dominant Alzheimer's disease, underscoring the similarities between both conditions (Supplementary Figure S7).
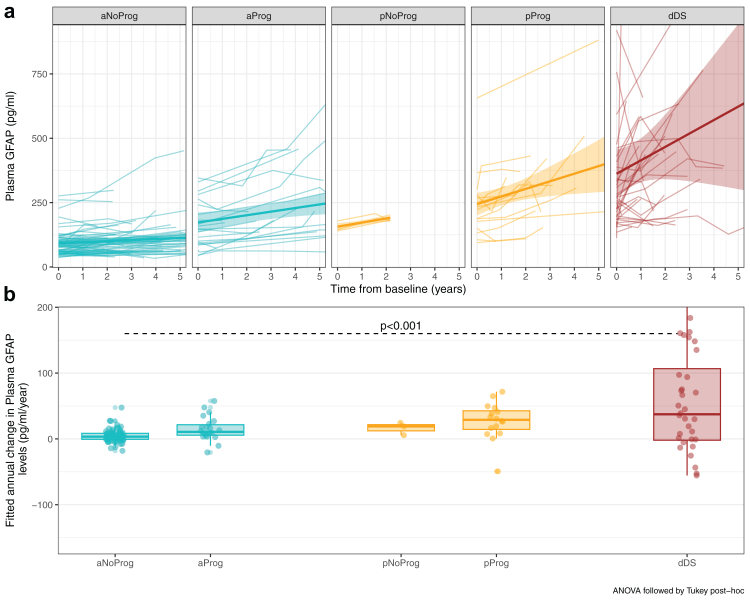
To study the prognostic value of plasma GFAP, asymptomatic Down syndrome individuals and prodromal Alzheimer's disease patients were subclassified into “progressors” and “non-progressors” (Supplementary Table S3 and Supplementary Figure S8). In the asymptomatic group, progressors had significantly higher levels of plasma GFAP than non-progressors (1.8-fold change, age-adjusted ANCOVA post-hoc p < 0.001, Fig. 2a). We analysed the association of baseline plasma GFAP concentrations with future cognitive decline (Fig. 2b). Down syndrome participants were divided into tertiles according to their baseline plasma GFAP levels and using the cognitively normal group tertile cutoffs: low (<60.5 pg/ml), medium (60.5–85.9 pg/ml) and high (>85.9 pg/ml). High plasma GFAP concentrations were associated with a greater risk of cognitive status change compared with low GFAP (also when analysis was restricted by age, data not shown). Separated Kaplan-Meyer curves for asymptomatic and prodromal participants are plotted in Supplementary Figure S9. The hazard ratio (HR) of plasma GFAP (HR = 2.99; 95% CI, 1.61–5.56) was higher than that of NfL (HR = 2.02; 95% CI, 1.05–3.89) and p-tau181 (HR = 2.63; 95% CI, 1.55–4.48) (Supplementary Figure S10).
Fig. 2.
Association of clinical progression and plasma GFAP levels. (a) Asymptomatic and prodromal Alzheimer's disease participants were classified into progressors (Prog) or non-progressors (NoProg) to Alzheimer's disease dementia. Data were analyzed using ANCOVA followed by Tukey post-hoc analysis. Only significant associations are shown. (b) Kaplan Meier curves of clinical progression. Asymptomatic participants were classified into tertiles according to their plasma GFAP levels: blue lines show the lowest tertile (below 60.5 pg/ml), green lines show the medium tertile (between 60.5 and 85.9 pg/ml), and red lines show the highest tertile (above 85.9 pg/ml).
We next studied the longitudinal changes of plasma GFAP. A subset of 420 participants with Down syndrome (288 asymptomatic, 48 prodromal and 84 Alzheimer's disease dementia) had two or more plasma samples available for analysis. Supplementary Table S4 includes the information of participants with longitudinal samples available. Using linear-mixed models, we found that plasma GFAP concentrations showed a non-significant longitudinal increase of 3.7% per year (−2.3 to 10.3%) in asymptomatic non-progressors, 7.1% (−3.4 to 24%) in asymptomatic progressors, 9.8% (−16.8 to 46.3%) in prodromal Alzheimer's disease non-progressors and 8.4% per year (−4.0 to 23.3%) in prodromal progressors; and a significant increase of 19.8% per year (11.8–33.0) in Alzheimer's disease dementia patients (Fig. 3a). However, when comparing the increase of the asymptomatic non-progressors with the other groups, we only found significant differences in the comparisons with Alzheimer's disease dementia patients (Fig. 3b). Similar results were found in sporadic Alzheimer's disease (Supplementary Figure S11 and Supplementary Table S5).
Fig. 3.
Trajectories (a) and estimation of the annual increase (b) in plasma GFAP concentrations across diagnostic categories in the Down syndrome population. Participants were grouped according to diagnostic at baseline: asymptomatic (a), prodromal Alzheimer's disease (p) and Alzheimer's disease dementia (d) and sub-classified into progressors (Prog) and non-progressors (NoProg) to Alzheimer's disease dementia. aNoProg: asymptomatic Down syndrome, non-progressor; aProg: asymptomatic Down syndrome, progressor; pNoProg; prodromal Alzheimer's disease in Down syndrome, non-progressor; pProg: prodromal Alzheimer's disease in Down syndrome, progressor; dDS: Alzheimer's disease dementia in Down syndrome.
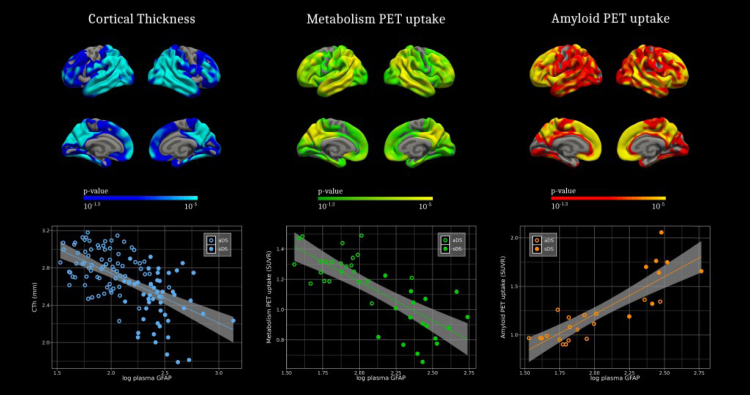
Finally, we analysed the correlations of plasma GFAP with other biomarkers of Alzheimer's disease pathology—neuroimaging and fluid biomarkers. Fig. 4 shows the regions with significant associations between plasma GFAP and cortical thinning, and Aβ PET in the whole Down syndrome. A negative correlation was found between cortical thickness and GFAP levels in typical Alzheimer's disease regions—the temporoparietal, precuneus, posterior cingulate, and frontal areas. Furthermore, GFAP levels were positively associated with amyloid deposition in the whole brain, especially significant in temporal, precuneus, and frontal regions. These associations remained after correcting for age and sex (Supplementary Figure S12). However, FDG levels did not correlate with plasma GFAP after adjusting for co-variates (Supplementary Figure S13). Importantly, autosomal dominant Alzheimer's disease presented a very similar pattern (Supplementary Figure S14). Of note, concentrations of plasma GFAP discriminated amyloid positive from amyloid negative individuals, both when stratified by amyloid PET and CSF Aβ42/40 ratio (Supplementary Figures S15 and S16). Associations between plasma biomarkers and their counterparts in CSF (Supplementary Figure S17) showed high correlations for NfL (Spearman rho = 0.81; p < 0.001) and p-tau181 (Spearman rho = 0.71; p < 0.001), but lowest for GFAP (Spearman rho = 0.64; p < 0.001) in the Down syndrome participants. Plasma GFAP was, nonetheless, highly correlated with CSF Aβ42/40 ratio (Spearman rho = −0.7; p < 0.001) and with amyloid PET centiloid (Spearman rho = 0.77; p < 0.001). However, plasma GFAP had moderate associations with both CSF total-tau (Spearman rho = 0.58; p < 0.001) and CSF YLK-40, another biomarker of astrocyte reactivity (Spearman rho = 0.53; p < 0.001).
Fig. 4.
Association of plasma GFAP levels with neuroimaging biomarkers in Down syndrome. Association of GFAP levels with cortical thickness measured by MRI (up); and amyloid PET (down) in the Down syndrome population. The p-value used was 0.05 for uncorrected data. A Monte Carlo simulation was applied as implemented in Freesurfer. This cluster-based method performs 10,000 simulations to generate a distribution of maximum cluster size generated by random noise. Only clusters that were larger than those obtained during the Monte Carlo simulation more than 500 iterations (corrected alpha threshold of 0.05) were considered to survive multiple comparisons.
Discussion
This longitudinal study assessed the potential of plasma and CSF GFAP as a diagnostic and prognostic biomarker for Alzheimer's disease in Down syndrome and compared the dynamics of this biomarker with both autosomal dominant and sporadic Alzheimer's disease. Plasma GFAP showed the earliest increases and the largest fold-change in the dementia stage. The magnitude and temporality of changes were similar in autosomal dominant Alzheimer's disease and larger than in sporadic Alzheimer disease. CSF GFAP levels, on the other hand, presented the lowest fold changes. Altogether our results support the use of plasma GFAP in clinical practice and clinical trials.
The early elevations in plasma GFAP levels from mid-late 20s (more than 20 years before symptom onset) in parallel with the reported decreases in CSF Aβ levels is one of the main findings of this paper. They occur ten years before plasma p-tau181 elevations, which start to raise in parallel with the increases in amyloid PET uptake. Therefore, although both these biomarkers have been shown to reflect amyloid pathology in early preclinical Alzheimer's disease, our data indicates that GFAP increases occur earlier at an age in which diffuse plaques predominate and in parallel with CSF Aβ changes4 Plasma p-tau181 elevations start 10 years later when amyloid PET uptake begin to increase, more in relation to neuritic plaques.3,4 Importantly, the temporality of the biomarker changes was coincident in Down syndrome and autosomal dominant Alzheimer's disease, underscoring the similarities between both forms of genetically determined Alzheimer's disease.1,3,4 In addition, plasma GFAP levels seemed to increase continuously along the Alzheimer's disease continuum, so although the earliest to change, it also was the biomarker with the highest AUC to discriminate symptomatic from asymptomatic participants in the three variants of Alzheimer's disease.
In addition to its diagnostic performance, plasma GFAP also predicts clinical progression along the Alzheimer's disease continuum in adults with Down syndrome, in agreement with previous studies, which reported that plasma GFAP predicts future conversion to Alzheimer's disease dementia in cognitively normal26 and mild cognitively impaired15 individuals. Here, we show that plasma GFAP concentrations were significantly different between asymptomatic progressors and non-progressors, and participants with high plasma GFAP levels at baseline were three times more likely to experience a cognitive change in the following five years. In addition, the longitudinal trajectories of plasma GFAP showed a nominal increase (with relatively large variance) along the Alzheimer's disease continuum in the rate of change in plasma GFAP concentration, but it was not significant in asymptomatic and prodromal Alzheimer's disease individuals (but did increase of almost 20% per year in participants at the dementia stage).
In contrast with the excellent diagnostic and prognostic performances of plasma GFAP, CSF GFAP was the biomarker with the lowest accuracy to diagnose or predict symptomatic Alzheimer's disease. Studies in the past assessing CSF GFAP in sporadic Alzheimer's disease have reported inconsistent results.27 Recently, two publications showed elevated concentrations of plasma GFAP with disease progression and amyloid deposition, but no significant differences in the CSF GFAP levels across clinical Alzheimer's disease continuum13 or groups according to amyloid PET status.14 This discrepancy between the diagnostic and prognostic performances of plasma and CSF GFAP levels remains unresolved. It has been proposed that given that astrocytes are key components in the maintenance of the neurovascular unit and the blood–brain barrier,28 their dysfunction could release GFAP directly to the bloodstream explaining the elevations in plasma GFAP, but not in CSF. Other plausible causes could be differences in stability or fragmentation patterns of GFAP in blood and CSF. On one hand, blood GFAP levels have been shown to remain stable for up to 7 freeze-thaw cycles, while CSF GFAP signal decreases with increasing freeze-thawing.29 On the other, the GFAP antibody in the immunoassay used in the current study has been shown to detect 50–38 kDa GFAP bands that resemble calpain-cleaved GFAP.30 It could be that GFAP fragmentation pattern is different in CSF and blood, and the antibody has major affinity for the fragments present in blood.
To further investigate the link between plasma GFAP and Alzheimer's disease pathophysiology, we decided to study its association with established markers of Alzheimer's disease. We found that plasma GFAP was correlated with cortical thinning and amyloid PET uptake in both Down syndrome and autosomal dominant Alzheimer's disease. In a recent study, plasma GFAP and p-tau217 were the two only plasma biomarkers able to predict Alzheimer's disease pathology as measured by PET in individuals with Down syndrome even when models were covaried for age.21 Here we also report that plasma GFAP levels are significantly increased in Aβ positive compared to Aβ negative individuals in Down syndrome. This link between amyloid deposition and reactive astrocytes has also been shown in sporadic Alzheimer's disease. Several papers have found that plasma GFAP levels distinguish amyloid positive from amyloid negative (PET or CSF Aβ42/40 ratio)13, 14, 15,26 individuals; correlate with higher amyloid PET13,14; and positively associate with tau pathology only among individuals with concomitant Aβ pathology.13 Studies conducted with novel astrocytic PET tracers have shown that reactive astrocytes follow the same spatial distribution and are closely associated with amyloid plaques in the brain.31 Altogether these results suggest that amyloid deposition induces a phenotypic change of astrocytes from early stages in Alzheimer's disease that results in increased plasma GFAP levels. We have already discussed the different temporality of plasma GFAP and p-tau181 taking advantage of the unique opportunity of studying autosomal dominant and Down syndrome. In this line, we found that GFAP was more closely associated to CSF Aβ than CSF tau, but that CSF YKL40 levels (another biomarker of astrogliosis32 correlated more closely with CSF p-tau181). Interestingly, a recent article reported that while GFAP levels are associated with Aβ-PET but not tau-PET load, YKL-40 levels are associated with elevated tau-PET but not Aβ-PET burden,33 suggesting the existence of distinct astrocyte biomarker signatures in response to brain Aβ and tau accumulation. We speculate that both these biomarkers reflect astrogliosis, but this reaction might be a response to different pathological mechanisms.
Our findings have direct implications in the management of Alzheimer's disease in Down syndrome and in clinical trials. The diagnosis of symptomatic Alzheimer's disease in this population is complex because it requires a demonstration of deterioration from the neurodevelopmental intellectual disability. Plasma GFAP was the most accurate biomarker to discriminate symptomatic from asymptomatic participants. Eventually, plasma GFAP levels could be included in a panel of plasma biomarkers to screen for symptomatic Alzheimer's disease (or those at higher risk for) in Down syndrome. Beyond its use in clinical settings, plasma GFAP has great potential for recruitment and monitoring in clinical trials. The early changes in plasma GFAP, in parallel to CSF Aβ and ten years prior to amyloid PET positivity, and its high prognostic value make it an ideal candidate to select individuals in preventive trials. In addition, its strong link to amyloid brain pathology supports its use for target engagement, as demonstrated in the TRAILBLAZER-ALZ clinical trial.34 Plasma GFAP levels were significantly lower with donanemab treatment and significantly correlated with the Centiloid percent change in amyloid following therapy.
The major strength of the current study is our multi-centre large cohort of adults with Down syndrome with longitudinal measurements and comprehensive clinical follow-up. Furthermore, we provide the quantification of plasma and CSF p-tau181 and NfL in this population and in comparison, to euploid controls asymptomatic and symptomatic for sporadic Alzheimer's disease and autosomal dominant Alzheimer's disease. However, this study presents some limitations. Firstly, the clinical diagnosis of cognitive decline in Down syndrome, especially in prodromal stages is particularly challenging, which might have affected the diagnosis in the asymptomatic and symptomatic groups. The estimated age of prodromal Alzheimer's disease in Down syndrome for statistical analysis was set to 53.8, but this value could be different in another sample set. Although proven useful in several prior studies, the concept of estimated years to symptom onset is relatively new in this population.3,7,22 Secondly, the difference in the sample size between Down syndrome and sporadic Alzheimer's disease, as well as between the plasma and the CSF samples available, limited the comparisons. Finally, the statistical analysis of our longitudinal results could have been affected by low sample size in some groups, a relatively limited follow-up period in some participants and dispersion in the levels of plasma GFAP (and the longitudinal trajectory).
In summary, we show the utility of plasma GFAP as a biomarker of Alzheimer's disease in Down syndrome. Its high diagnostic accuracy, prognostic value, and association with amyloid PET, suggest this biomarker could be used in patient care and clinical trials.
Contributors
LMG, DA, NJA, HZ and JF created the concept and design. Data acquisition and analysis was performed by LMG, DA, NJA, JP, JLR and JF. JL, BB (Bosch), JLR, JLR, MCI, OW, MB, PRK, IB, AL (Lladó), WSB, LV, FGO, BB (Benejam), JJAM, TKK, GN, AB, ALB, RB, AL (Lleó), KB and RSV contributed to the sample selection/and or interpretation of the data. LMG, DA and JF verified the underlying data. LMG, DA, NJA, JP, HZ and JF drafted the manuscript and all authors revised. All authors read and approved the final manuscript.
Data sharing statement
Bulk anonymized data can be shared by request from qualified investigators, providing data transfer agrees with EU legislation and decisions by the institutional review board of each participating center.
Declaration of interests
JF has served as a consultant for Novartis and Lundbeck, has received honoraria for lectures from Roche, Novo Nordisk, Laboratorios Carnot, Nestle, Esteve and Biogen and served at advisory boards for AC Immune, Alzheon, Zambon and Lundbeck. DA participated in advisory boards from Fujirebio-Europe and Roche Diagnostics and received speaker honoraria from Fujirebio-Europe, Roche Diagnostics, Nutricia, Krka Farmacéutica S.L., Zambon S.A.U. and Esteve Pharmaceuticals S.A. JF, DA and ALL declare a filed patent sapplication (WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease). NJA has given lectures in sponsored symposia by Roche and Eli Lily. KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. AL has served at scientific advisory boards from Fujirebio-Europe, Nutricia, Roche-Genentech, Biogen, Grifols and Roche Diagnostics and has filed a patent application of synaptic markers in neurodegenerative diseases (WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease). HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
Acknowledgments
We thank all the participants with Down syndrome, their families, and their carers for their support of, and dedication to this research. We also acknowledge Fundació Catalana Síndrome de Down for global support and the members of the Alzheimer Down Unit and the Memory Unit from Hospital de la Santa Creu i Sant Pau for their daily work and dedication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104547.
Appendix A. Supplementary data
References
- 1.Iulita M.F., Chavez D.G., Christensen M.K., et al. Association of Alzheimer disease with life expectancy in people with Down syndrome. JAMA Netw Open. 2022;5(5) doi: 10.1001/jamanetworkopen.2022.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard C., Mobley W., Hardy J., Williams G., Corbett A. Dementia in Down's syndrome. Lancet Neurol. 2016;15(6):622–636. doi: 10.1016/S1474-4422(16)00063-6. [DOI] [PubMed] [Google Scholar]
- 3.Fortea J., Vilaplana E., Carmona-Iragui M., et al. Clinical and biomarker changes of Alzheimer's disease in adults with Down syndrome: a cross-sectional study. Lancet. 2020;395(10242):1988–1997. doi: 10.1016/S0140-6736(20)30689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortea J., Zaman S.H., Hartley S., Rafii M.S., Head E., Carmona-Iragui M. Alzheimer's disease associated with Down syndrome: a genetic form of dementia. Lancet Neurol. 2021;20(11):930–942. doi: 10.1016/S1474-4422(21)00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benejam B., Videla L., Vilaplana E., et al. Diagnosis of prodromal and Alzheimer's disease dementia in adults with Down syndrome using neuropsychological tests. Alzheimers Dement (Amst) 2020;12(1) doi: 10.1002/dad2.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack C.R., Jr., Bennett D.A., Blennow K., et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortea J., Carmona-Iragui M., Benejam B., et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer's disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol. 2018;17(10):860–869. doi: 10.1016/S1474-4422(18)30285-0. [DOI] [PubMed] [Google Scholar]
- 8.Karikari T.K., Pascoal T.A., Ashton N.J., et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422–433. doi: 10.1016/S1474-4422(20)30071-5. [DOI] [PubMed] [Google Scholar]
- 9.Ashton N.J., Pascoal T.A., Karikari T.K., et al. Plasma p-tau231: a new biomarker for incipient Alzheimer's disease pathology. Acta Neuropathol. 2021;141(5):709–724. doi: 10.1007/s00401-021-02275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmqvist S., Janelidze S., Quiroz Y.T., et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772–781. doi: 10.1001/jama.2020.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashton N.J., Leuzy A., Lim Y.M., et al. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Commun. 2019;7(1):5. doi: 10.1186/s40478-018-0649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashton N.J., Janelidze S., Al Khleifat A., et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12(1):3400. doi: 10.1038/s41467-021-23620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedet A.L., Milà-Alomà M., Vrillon A., et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 2021;78(12):1471–1483. doi: 10.1001/jamaneurol.2021.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira J.B., Janelidze S., Smith R., et al. Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer's disease. Brain. 2021;144(11):3505–3516. doi: 10.1093/brain/awab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicognola C., Janelidze S., Hertze J., et al. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimer's Res Ther. 2021;13(1):68. doi: 10.1186/s13195-021-00804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connor A., Abel E., Benedet A.L., et al. Plasma GFAP in presymptomatic and symptomatic familial Alzheimer's disease: a longitudinal cohort study. J Neurol Neurosurg Psychiatry. 2022;94(1):90–92. doi: 10.1136/jnnp-2022-329663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsipis G., Tzekaki E.E., Tsolaki M., Pantazaki A.A. Salivary GFAP as a potential biomarker for diagnosis of mild cognitive impairment and Alzheimer's disease and its correlation with neuroinflammation and apoptosis. J Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577744. [DOI] [PubMed] [Google Scholar]
- 18.Bettcher B.M., Olson K.E., Carlson N.E., et al. Astrogliosis and episodic memory in late life: higher GFAP is related to worse memory and white matter microstructure in healthy aging and Alzheimer's disease. Neurobiol Aging. 2021;103:68–77. doi: 10.1016/j.neurobiolaging.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lleó A., Zetterberg H., Pegueroles J., et al. Phosphorylated tau181 in plasma as a potential biomarker for Alzheimer's disease in adults with Down syndrome. Nat Commun. 2021;12(1):4304. doi: 10.1038/s41467-021-24319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bejanin A., Iulita M.F., Vilaplana E., et al. Association of apolipoprotein E ϵ4 allele with clinical and multimodal biomarker changes of Alzheimer disease in adults with down syndrome. JAMA Neurol. 2021;78(8):937–947. doi: 10.1001/jamaneurol.2021.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janelidze S., Christian B.T., Price J., et al. Detection of brain tau pathology in down syndrome using plasma biomarkers. JAMA Neurol. 2022;79(8):797–807. doi: 10.1001/jamaneurol.2022.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmona-Iragui M., Alcolea D., Barroeta I., et al. Diagnostic and prognostic performance and longitudinal changes in plasma neurofilament light chain concentrations in adults with Down syndrome: a cohort study. Lancet Neurol. 2021;20(8):605–614. doi: 10.1016/S1474-4422(21)00129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janelidze S., Bali D., Ashton N.J., et al. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer’s disease. Brain. 2022:awac333. doi: 10.1093/brain/awac333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteba-Castillo S., Dalmau-Bueno A., Ribas-Vidal N., Vilà-Alsina M., Novell-Alsina R., García-Alba J. Adaptation and validation of CAMDEX-DS (Cambridge examination for mental disorders of older people with down's syndrome and others with intellectual disabilities) in Spanish population with intellectual disabilitiesRev Neurol. 2013;57(8):337–346. [PubMed] [Google Scholar]
- 25.Nübling G., Loosli S.V., Wlasich E., et al. A German version of the Cambridge examination for mental disorders of older people with Down's syndrome and others with intellectual disabilities: a diagnostic procedure for detecting dementia in people with Down's syndromeZ Gerontol Geriatr. 2020;53(6):546–551. doi: 10.1007/s00391-019-01591-7. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee P., Pedrini S., Stoops E., et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer's disease. Transl Psychiatry. 2021;11(1):27. doi: 10.1038/s41398-020-01137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson B., Lautner R., Andreasson U., et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 28.Carter S.F., Herholz K., Rosa-Neto P., Pellerin L., Nordberg A., Zimmer E.R. Astrocyte biomarkers in Alzheimer's disease. Trends Mol Med. 2019;25(2):77–95. doi: 10.1016/j.molmed.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Simrén J., Weninger H., Brum W.S., et al. Differences between blood and cerebrospinal fluid glial fibrillary acidic protein levels: the effect of sample stability. Alzheimers Dement. 2022;18(10):1988–1992. doi: 10.1002/alz.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoltewicz J.S., Scharf D., Yang B., Chawla A., Newsom K.J., Fang L. Characterization of antibodies that detect human GFAP after traumatic brain injury. Biomark Insights. 2012;7:71–79. doi: 10.4137/BMI.S9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Vieitez E., Saint-Aubert L., Carter S.F., et al. Diverging longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer's disease. Brain. 2016;139(Pt 3):922–936. doi: 10.1093/brain/awv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Querol-Vilaseca M., Colom-Cadena M., Pegueroles J., et al. YKL-40 (Chitinase 3-like I) is expressed in a subset of astrocytes in Alzheimer's disease and other tauopathies. J Neuroinflammation. 2017;14(1):118. doi: 10.1186/s12974-017-0893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrari-Souza J.P., Ferreira P.C.L., Bellaver B., et al. Astrocyte biomarker signatures of amyloid-β and tau pathologies in Alzheimer's disease. Mol Psychiatry. 2022;27(11):4781–4789. doi: 10.1038/s41380-022-01716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pontecorvo M.J., Lu M., Burnham S.C., et al. Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: a secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79(12):1250–1259. doi: 10.1001/jamaneurol.2022.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.