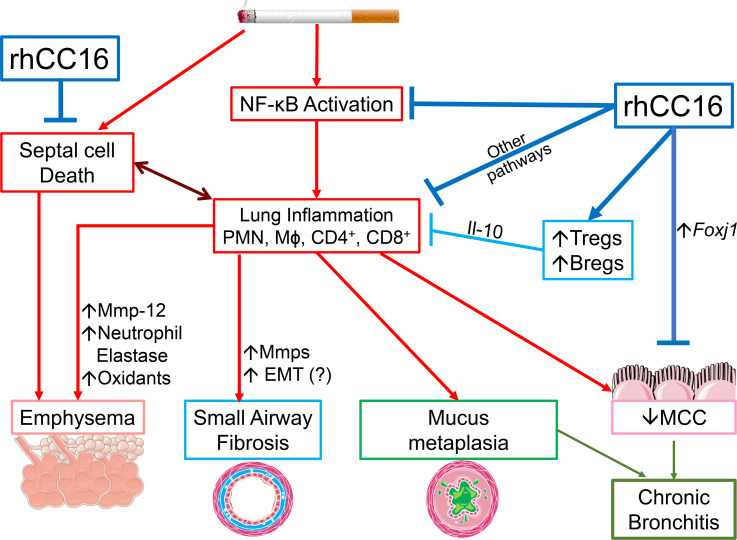
Figure 12. Beneficial effects of rhCC16 on COPD-like disease in CS-exposed mice.
Exposing WT and Cc16–/– mice to CS induces a chronic pulmonary inflammatory response (with increases in PMN, macrophage [Mɸ], and CD4+ and CD8+ lymphocyte counts) mediated, in part, by increased NF-κB activation in the lungs. These leukocytes (and activated epithelial cells) release metalloproteinases (Mmps), and/or neutrophil elastase (NE), other proteinases, oxidants, and growth factors that promote emphysema development and SAF, and/or increase Muc5ac and Muc5b expression in airway epithelial cell (mucus metaplasia). CS also promotes emphysema development by increasing alveolar septal cell apoptosis. CS itself and increased levels of PMN-derived NE and Muc5b in CS-exposed airways impair mucociliary clearance (MCC). Epithelial cell mucus metaplasia and impaired MCC contribute to CB-like disease in CS-exposed mice. Delivering rhCC16 to the lungs of CS-exposed mice limits the progression of CS-induced emphysema development, SAF, and CB-like disease in WT and Cc16–/– mice likely by reducing the pulmonary inflammatory response to CS (in part by reducing the exaggerated NF-κB activation in CS-exposed Cc16–/– lungs) and by reducing alveolar septal cell apoptosis. Treating mice with rhCC16 also reduces pulmonary inflammation by increasing Treg and Breg accumulation the lungs, which release antiinflammatory Il-10. The reduction in inflammation induced by rhCC16 may reduce septal cell death and loss of parenchymal architecture. In addition, rhCC16 may limit the progression of CB-like disease in mice by improving MCC by reducing airway mucus cell metaplasia and increasing the expression of Foxj1, which is required for the generation of motile cilia on epithelial cells. EMT, epithelial-mesenchymal transition.