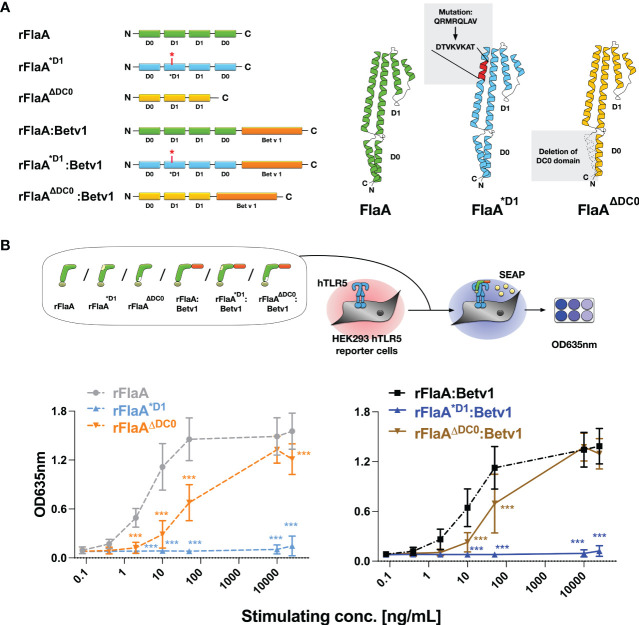
Figure 2.
Generation and characterization of recombinant FlaA- and rFlaA:Betv1-mutants replacing either the TLR-activating sequence stretch in the D1 domain or truncating the C-terminal D0 domain. To investigate the mechanism of rFlaA:Betv1-mediated macrophage activation, four mutant proteins were generated (A). For the generation of rFlaA*D1 and rFlaA*D1:Betv1 eight amino acids of the flagellin D1 structural domain (QRMRQLAV) reported to contribute to recognition of flagellin by TLR5 were replaced with the sequence DTVKVKAT (A). For rFlaAΔDC0 and rFlaAΔDC0:Betv1 the flagellin DC0 domain was deleted (A). The ability of wild-type and protein mutants to activate human TLR5 was tested using HEK-Blue™ hTLR5 reporter cells (B). Data are the mean results of three independent experiments ± SD. Statistical comparisons were performed between the wild-type proteins (rFlaA or rFlaA:Betv1) and respective mutants treated with equimolar stimulation doses and statistical significance was shown as ***: p-value < 0.001.