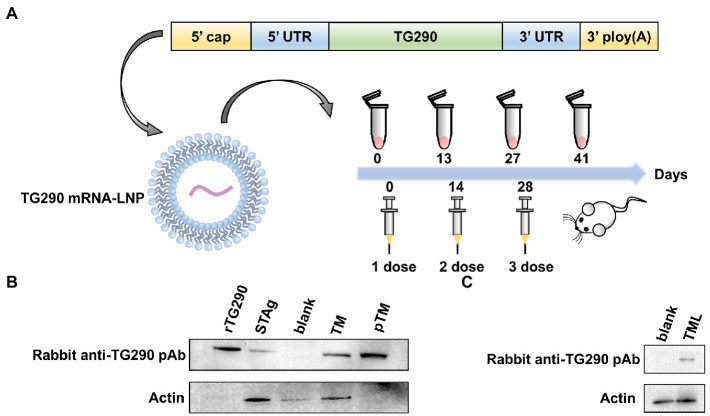
Figure 2.
The construction, protein expression and delivery of TG290 mRNA-LNP vaccine. (A) The engineered mRNA construct. An mRNA encoding the TG290 was designed with 5′ and 3′ untranslated regions (UTRs) flanking the coding sequence, a 5′ cap-1 structure and a 3′ poly(A) tail. In vitro-synthesized mRNA encapsulated in a lipid nanoparticle to be applied in in vitro and in vivo experiments. (B) The expression of TG290 in HEK293 cell lysates and STAg was determined by Western blot. rTG290 served as a positive control; STAg: (soluble antigen of T. gondii); Blank: non-transfected cells; Unpurified cell lysate from TG290 mRNA-transfected cells ©; Purified supernatant from TG290 mRNA-transfected cells (pTM). (C) In vitro-synthesized TG290 mRNA was encapsulated in a lipid nanoparticle and administered to C2C12 cells. Lysate was analyzed by Western blotting with Rabbit anti-TG290 pAb. Blank: empty transfected cells; TG290 mRNA-LNP transfected cells (TML).