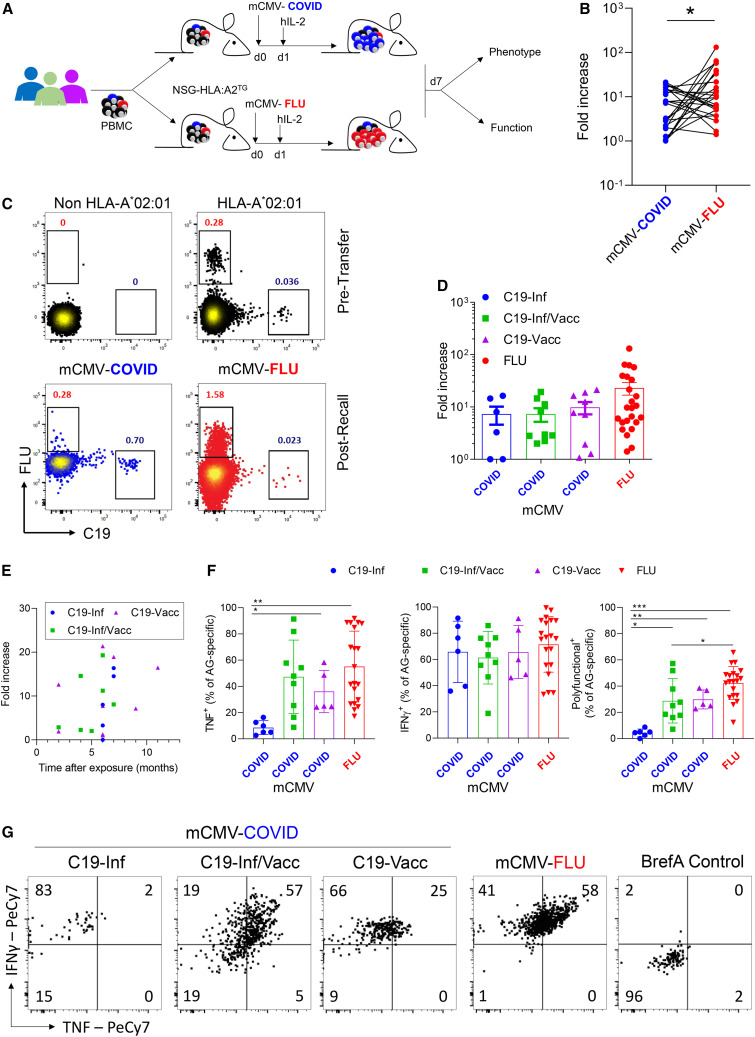
Figure 4.
Vaccination-induced memory cells have increased functional potential after in vivo recall
PBMCs were analyzed pretransfer for the frequency of tetramer+ cells. Cells were divided into two equal fractions and transferred to NSG-HLA:A∗02 transgenic mice. On the same day, animals were infected i.p. with 2 × 105 PFU mCMV-COVID or mCMV-FLU. The next day, mice were injected i.p. with human IL-2 (100 ng/mouse). 7 days post-infection, donor cells in peritoneal exudate cells (PECs) were analyzed (n = 25).
(A) Experimental setup.
(B) Line graph showing expansion of cells of the same donor as fold increase over pretransfer, comparing C19- with FLU-specific CD8+ T cells.
(C) Representative FACS plots of CD8+ T cell stained with HLA-A∗02 tetramers pretransfer or 7 days post-infection. Non-HLA-A∗02 cells are included as negative control. Numbers indicate percentages. Gated is for live hCD45+hCD8+ cells.
(D) Fold increase of antigen-specific cells segregated by C19 groups.
(E) Correlation between time after last antigen exposure and the expansion of virus-specific cells after mCMV-C19 infection.
(F–G) On day 7 post-infection, PECs were re-stimulated in vitro with PMA/IONO for 4 h in the presence of Brefeldin A and analyzed by flow cytometry. Shown are (F) cytokine production of live hCD45+hCD8+tetramer+ cells and (G) representative FACS plots gated for hCD45+hCD8+tetramer+ cells. Indicated are means ± SEM.
p values were calculated using paired Student t test (B) and Kruskal-Wallis rank-sum test with Dunn’s post hoc test for multiple comparisons (F). ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
See also Figure S4.