Figure 1. Trial Design, Neurostimulation Device Lead and Stimulation Modes, and Electrode Localization.
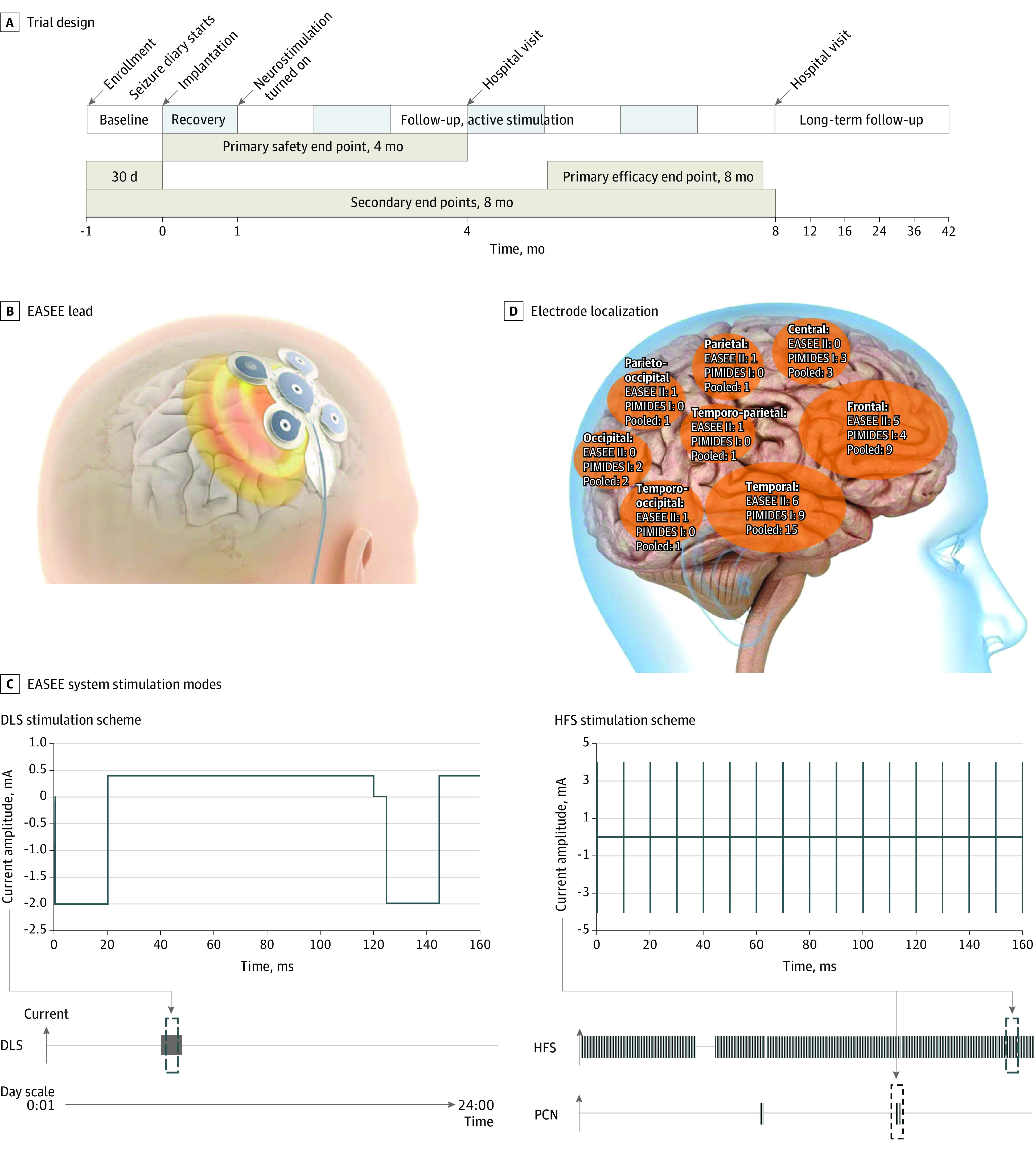
A, Trial time line for the A Pilot Study to Assess the Feasibility of Neurostimulation With the EASEE System to Treat Medically Refractory Focal Epilepsy (EASEE II) and A Pilot Study to Assess the Feasibility of Patient-Controlled Neurostimulation With the EASEE System to Treat Medically Refractory Focal Epilepsy (PIMIDES I) studies. B, The neurostimulation device consists of a lead placed epicranially above the respective epileptic focus and connected to the pulse generator in the chest. The lead includes a 5-channel pseudo-Laplace electrode (a central electrode surrounded by 4 peripheral electrodes; total diameter, 77 mm) and a connecting cable. C, Device stimulation modes are direct current–like stimulation (DLS; cathodal pulses of 2 mA, duration 20 microseconds, with equilibrating pulses of 100-microseconds’ duration for 20 minutes per day), high-frequency stimulation (HFS; bursts of 4 mA, rectangular biphasic symmetric, at 100 Hz, pulse width 160 microseconds; trains of 500-microseconds’ duration applied every 2 minutes), and on-demand patient-controlled neurostimulation (PCN) for 10 seconds to 60 seconds according to settings (available to PIMIDES I cohort). Stimulation intensity was based on finite element method modeling and experimental data from Liu et al8 (repetitive direct-current stimulation available in Lu et al9). D, Implantation of the device electrode was tailored to each patient and placed above the respective epileptic foci. Shown here is the distribution of electrode locations in the study population, projected to the right hemisphere.
