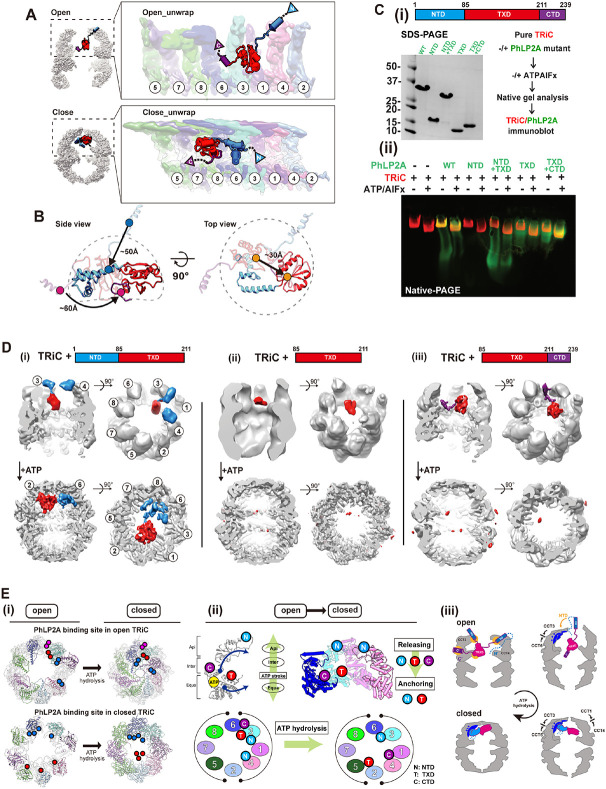
Figure 5. Domain-wise characteristics of PhLP2A in relationship with TRiC.
(A) Global rearrangement of PhLP2A in the TRiC folding chamber in the unwrapped view. (Top) Un wrapped view of PhLP2A in which NTD is in the expanded form in TRiC in the open conformation. (Bottom) Unwrapped view of PhLP2A in the closed conformation of TRiC. Note that PhLP2A NTD is compacted and constrained. (B) Conformational and orientation changes of PhLP2A in the open or c losed TRiC folding chamber. PhLP2A NTD undergoes an orientation change of about ~ 50 Å from e xtended outward to bent inward, closer to the TXD. The TXD is lifted about ~ 30 Å from the equat or to the apical contact through flipping but retains its conformation. The CTD moves ~ 60 Å followi ng the movement of TXD. (C) (i)(left) SDS-PAGE of PhLP2A wild-type (WT) and four truncated muta nt constructs of PhLP2A: NTD (aa 1-84), CTD truncation (aa 1-211, NTD+TXD), TXD (aa 85-211), N TD truncation (aa 85-239, TXD+CTD). (right) Schematic of the binding assay. (ii) Binding assay betw een TRiC and PhLP2A truncation mutants with or without ATP/AlFx. (D) CryoEM structures of three truncated mutants inside the TRiC in the open state and after ATP hydrolysis. (i) TRiC with NTD+T XD shows attributable NTD density at the apical domain with TXD and nTd+tXd orients like WT P hLP2A in closed TRiC. (ii) TRiC with TXD: attributable density of TXD at low resolution at the equat or, but no attributable density in closed TRiC. (iii) TRiC with TXD+CTD: CTD anchor shows high-res olution features like WT PhLP2A between CCT3/6 and TXD close to CCT3, but no attributable densi ty in closed TRiC. (E) (i) Schematic diagram of PhLP2A domain-wise interaction with the TRiC cham ber in open (left) and closed conformation (right). Colored circles indicate interacting residues in ope n TRiC and closed TRiC and their movements during the ATP cycle. Each PhLP2A domain is color coded as in (C). (ii) ATP hydrolysis event in CCT subunits cascades the releasing and re-anchoring process of phLP2A. N: NtD, T: TXD, C:CTD. (iii) Diagram of releasing of PhLP2A upon ATP-depen dent cycle of TRiC.