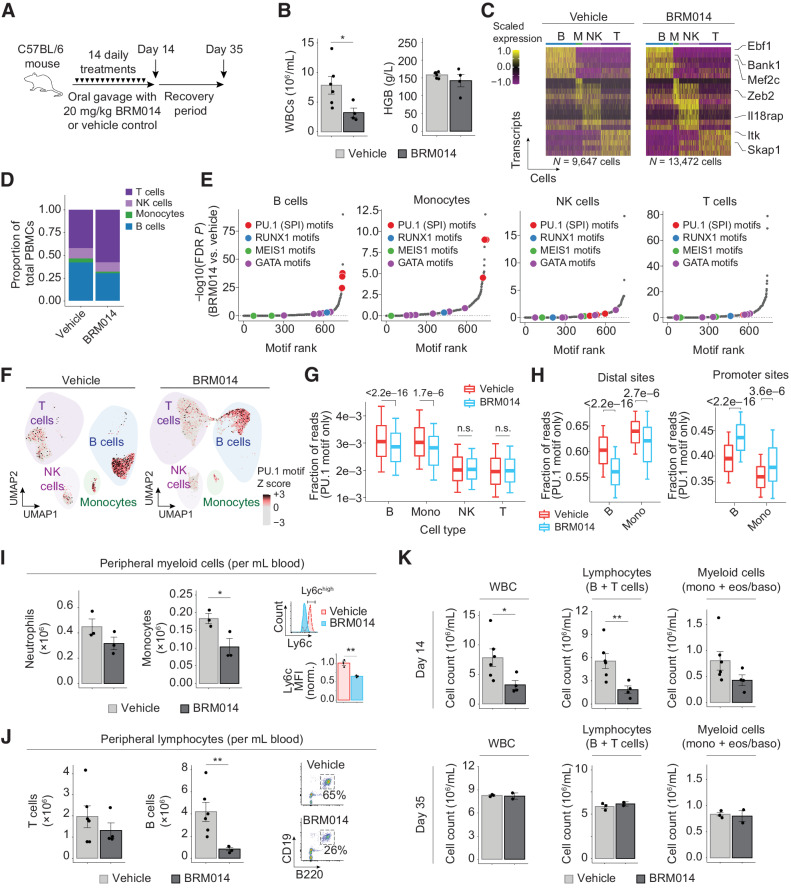
Figure 3.
SWI/SNF inhibition induces reversible leukopenia in PU.1-dependent nontumor peripheral blood lineages. A, Dosing scheme for in vivo treatment of healthy mice. B, WBC and hemoglobin (HGB) levels in peripheral blood at day 14. N = 6, vehicle; N = 4, BRM014. C, Cell type–specific transcriptional markers were unchanged by treatment, enabling consistent classification. D, Composition of nongranulocyte immune cell types in the peripheral blood of BRM014- and DMSO-treated mice. E, Ranking of significantly reduced differential TF motif accessibility by immune population from mice treated with BRM014 versus vehicle control. F, UMAP embedding of individual cells by snATAC profile, with overlayed PU.1 motif accessibility. G, Quantification of accessible PU.1 motifs across specific cell types. H, Shifts of accessible DNA sites containing PU.1 motifs in B cells and monocytes upon BRM014 treatment. I, Total number of peripheral neutrophils and monocytes at day 14 and Ly6c expression in circulating monocytes. N = 3 per condition. J, Total number of T and B cells at day 14, with B-cell gating strategy. N = 6, vehicle; N = 4, BRM014. K, Total WBC, lymphocyte, and myeloid cell counts at day 14 and day 35 (3 weeks posttreatment) measured by CBC. Day 14, N = 6 (vehicle) and N = 4 (BRM014). Day 35, N = 3 (vehicle) and N = 2 (BRM014). Error bars, mean ± SEM; *, P < 0.05; **, P < 0.01; n.s., nonsignificant.