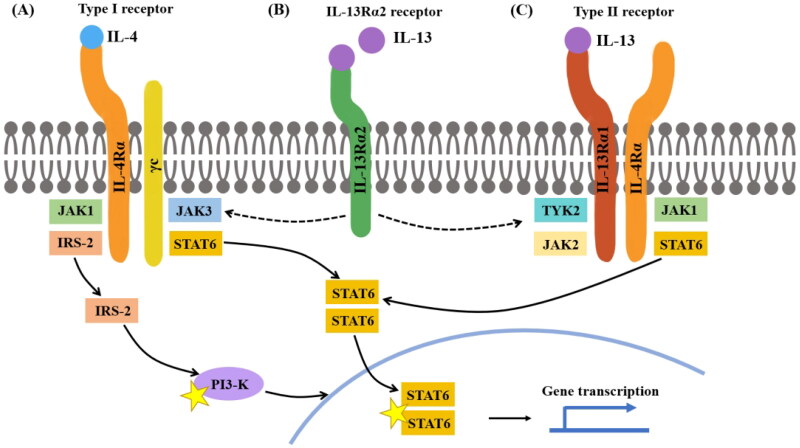
Figure 1.
IL-4 and IL-13 receptor structure IL-4 can bind to both type I and type II receptors. (A) Type I receptors consist of IL-4Rα and γc, whereas type II receptors consist of IL-4Rα and IL-13Rα1. When IL-4 binds to a type I receptor, Janus kinase (JAK) 1 and JAK3 are activated; both can induce tyrosine phosphorylation of the type I receptor intracellular domain, forming docking sites for downstream signaling molecules, such as signal transducer and activator of transcription (STAT) 6 and insulin receptor substrate-2 (IRS-2). Homodimers of STAT6 then translocate to the nucleus to facilitate transcription of IL-4- and IL-13-dependent genes. IRS-2 can activate signaling molecules, such as PI3K to induce gene transcription. (B) IL-13 binds to IL-13Rα2 with greater affinity than IL-13Rα1; the IL-13Rα2 receptor is considered a decoy receptor because it lacks a cytoplasmic signaling tail. However, the cytoplasmic domain of IL-13Rα2 may attenuate IL-4 signaling by inhibiting dimerization with γc or IL-13Rα1. (C) When IL-4 or IL-13 binds to a type II receptor, JAK1 and JAK2/TYK2 are activated.